Page 291 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 291
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
548 Metal Particles and Cluster Compounds
lated benzyne compound, is a good model for arene
activation. Other products of the Os 3 (CO) 12 + PPh 3 re-
action include HOs 3 (CO) 8 (PPh 2 C 6 H 4 ) and HOs 3 (CO) 7 -
(PPh 2 )(C 6 H 4 )(PPh 3 ).
It was stated earlier that the carbide in [HFe 4 (µ 4 -C)
(CO) 12 ] − is sufficiently exposed to allow direct attack
at the (µ 4 -C). Formation of a methyne (CH) ligand can
be achieved by prolonged treatment of the carbide clus-
ter with HCl yielding the neutral HFe 4 (µ 4 -CH)(CO) 12 .
More significantly, perhaps, is an alternate route to this
same methyne cluster compound. The dianion [Fe 4 (µ 4 -C)
(CO) 12 ] ,whenoxidizedbyAgBF 4 inthepresenceofH 2 ,
2−
yields HFe 4 (µ 4 -CH)(CO) 12 . The cluster–surface analogy
draws strength from this hydrogenation of a surfacelike
carbide atom from H 2 .
Fischer–Tropsch chemistry, involving the reduction of
CO by H 2 to yield hydrocarbons and some oxygen-
FIGURE 41 Structure of Os 3 (CO) 8 (PPh 2 )Ph(PPhC 6 H 4 ). Note containing products (alcohols, ketones, and aldehydes)
the µ 3 bonding of the (PPhC 6 H 4 ) ligand and the two-electron, has been postulated to proceed via the dissociative
three-center bond interaction with the C 6 H 5 ligand.
chemisorption of CO on a metal surface. The carbide
atoms may be hydrogenated, combine to form the back-
In the production of Os 3 (CO) 8 (PPh 2 )-(Ph)(PPhC 6 H 4 ) bone of larger chain hydrocarbon, or react with incoming
(Fig. 41) all three Os atoms become involved. All three CO. Total hydrogenation will yield methane, and partial
edges of the Os 3 core are bridged in this product. Note the
hydrogenation produces surface-bound alkane fragments.
presence of the (PPhC 6 H 4 ) ligand. It is very similar to the
The selectivity of Fischer–Tropsch synthesis for produc-
(PPh 2 C 6 H 4 ) ligand of the previously mentioned cluster.
ing n-alkanes in much greater yields than branched alka-
It is similar in that an ortho CH bond in a C 6 H 5 group
nes suggests that hydrogenation of carbides to CH 2 units
has oxidatively added to an adjacent Os atom forming an-
must be an important process. The presence of oxygen-
other five member ring. There is, however, one less phenyl
containing compounds suggests that CO may insert into
group in this ligand. The loss of a phenly group is com-
metal–carbide or metal–alkyl fragments. The diversity of
pensated for by the phosphorus forming a bond to the third
Fischer–Tropschchemistryhasbeenresponsibleformany,
Os atom. Ultimately this ligand achieves µ 3 -coordination.
some seemingly contradictory, mechanisms to be postu-
The second Os–Os edge is bridged by a C 6 H 5 ligand. This
lated. It seems probable that more than one mechanism
is a unique two-electron–three-center bond between a car-
is operative during the synthesis. We have to this point
bon of C 6 H 5 and an Os–Os edge. The near planar C 6 H 5
discussed a number of related Fe 4 C cluster compounds
ring lies nearly orthogonal to the Os Os bond which it
which provide the cluster analogs of proposed Fischer–
bridges. The third Os–Os edge is simply bridged by the
Tropsch intermediates. Figure 43a shows the proposed
phosphorus of PPh 2 .
surface-bound intermediates, and Fig. 43b shows the iso-
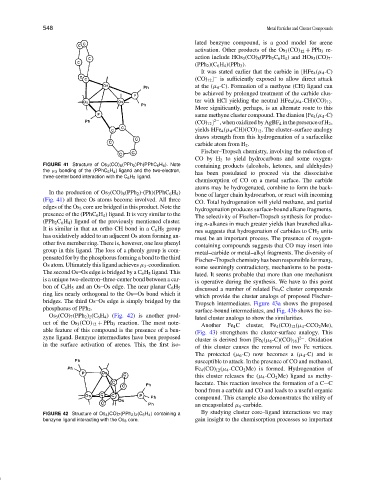
Os 3 (CO) 7 (PPh 2 ) 2 (C 6 H 4 ) (Fig. 42) is another prod-
lated cluster analogs to show the similarities.
uct of the Os 3 (CO) 12 + PPh 3 reaction. The most note-
Another Fe 4 C cluster, Fe 4 (CO) 12 (µ 4 -CCO 2 Me),
able feature of this compound is the presence of a ben-
(Fig. 43) strengthens the cluster-surface analogy. This
zyne ligand. Benzyne intermediates have been proposed 2−
cluster is derived from [Fe 6 (µ 6 -C)(CO) 16 ] . Oxidation
in the surface activation of arenes. This, the first iso-
of this cluster causes the removal of two Fe vertices.
The protected (µ 6 -C) now becomes a (µ 4 -C) and is
susceptible to attack. In the presence of CO and methanol,
Fe 4 (CO) 12 (µ 4 -CCO 2 Me) is formed. Hydrogenation of
this cluster releases the (µ 4 -CO 2 Me) ligand as methy-
lacetate. This reaction involves the formation of a C C
bond from a carbide and CO and leads to a useful organic
compound. This example also demonstrates the utility of
an encapsulated µ 6 -carbide.
FIGURE 42 Structure of Os 3 (CO) 7 (PPh 2 ) 2 (C 6 H 4 ) containing a By studying cluster core–ligand interactions we may
benzyne ligand interacting with the Os 3 core. gain insight to the chemisorption processes so important