Page 288 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 288
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
Metal Particles and Cluster Compounds 545
octahedral core. The square-based pyramid [Fe 5 (µ 5 -C)
(CO) 15 ] has a Group V counterpart in [HFe 5 (µ 5 -N)
(CO) 14 ]. The butterfly geometry of [HFe 4 (µ 4 -C) (CO) 12 ] −
−
is observed in the nitrido cluster [Os 4 (µ 4 -N) (CO) 12 ] .
There are also differences to be noted upon changing
from Group IV to Group V atomic donor ligands. The
heavier Group V atoms (P, As, Sb) do occupy interstitial
bonding sites whereas carbon is the only Group IV atom
to do so. In addition, the tendency to occupy surface sites
increases as demonstrated by [(µ 3 -As)Co 3 (CO) 9 ] and
[(µ 3 -As) 2 Fe 3 (CO) 9 ]. The arsenic atoms cap the M 3 tri-
angles giving overall tetrahedral and trigonal bipyramid
geometries, respectively. When bonded in this fashion As FIGURE 36 Structure of Os 6 (µ 4 -S) 2 (CO) 17 . A pentagonal
is acting as a three-electron donor leaving two electrons bipyramid core is defined by an Os 5 S 2 unit. The sulfido ligands
occupy nonadjacent equatorial sites. One Os(CO) 4 group bridges
to reside on As as a lone pair.
an OS axial OS equatorial bond.
In order for an atomic donor ligand to be interstitially
bound, the cluster core cavity must be large enough to ac-
commodate the donor. Since P, As, and Sb are significantly The two µ 4 -sulfido ligands present in [Os 6 (µ 4 -S) 2
larger than N they are not found within octahedral cavities (CO) 17 ] are exposed surface-like atoms as is clearly de-
since this does not provide enough space. Closo structures picted in Fig. 36 the two µ 4 -sulfido ligands occupy the two
which do host the larger Group V atomic donors include equatorialnonadjacentverticesofapentagonalbipyramid.
the bicapped square antiprism of [Rh 10 (µ 8 -L)(CO) 22 ] 3− The five remaining vertices of the cluster core are occu-
(L P, As) and the icosahedron as observed in [Rh 12 (µ 12 - pied by Os atoms. This leaves one Os atom unaccounted
Sb)(CO) 27 ] . To further illustrate the necessity for a large for. An Os(CO) 4 unit bridges an axial to equatorial Os Os
3−
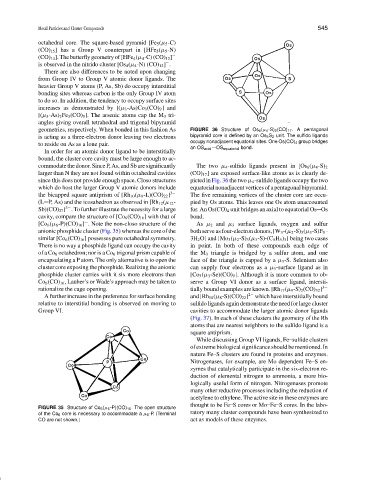
cavity, compare the structure of [Co 6 (CO) 16 ] with that of bond.
[Co 6 (µ 6 -P)(CO) 16 ] . Note the non-closo structure of the As µ 2 and µ 3 surface ligands, oxygen and sulfur
−
anionic phosphide cluster (Fig. 35) whereas the core of the both serve as four-electron donors, [W 3 -(µ 2 -S) 3 (µ 3 -S)F 9 ·
similar [Co 6 (CO) 16 ] possesses pure octahedral symmetry. 3H 2 O] and [Mo 3 (µ 2 -S) 3 (µ 3 -S)-(C 5 H 5 ) 3 ] being two cases
There is no way a phosphide ligand can occupy the cavity in point. In both of these compounds each edge of
ofa Co 6 octahedron; nor is a Co 6 trigonal prism capable of the M 3 triangle is bridged by a sulfur atom, and one
encapsulating a P atom. The only alternative is to open the face of the triangle is capped by a µ 3 -S. Selenium also
cluster core exposing the phosphide. Realizing the anionic can supply four electrons as a µ 3 -surface ligand as in
phosphide cluster carries with it six more electrons than [Co 3 (µ 3 -Se)(CO) 9 ]. Although it is more common to ob-
Co 6 (CO) 16 , Lauher’s or Wade’s approach may be taken to serve a Group VI donor as a surface ligand, intersti-
rationalize the cage opening. tially bound examples are known. [Rh 17 (µ 9 -S) 2 (CO) 32 ] 3−
A further increase in the preference for surface bonding and [Rh 10 (µ 8 -S)(CO) 22 ] 2− whichhaveinterstitially bound
relative to interstitial bonding is observed on moving to sulfido ligands again demonstrate the need for large cluster
Group VI. cavities to accommodate the larger atomic donor ligands
(Fig. 37). In each of these clusters the geometry of the Rh
atoms that are nearest neighbors to the sulfido ligand is a
square antiprism.
While discussing Group VI ligands, Fe–sulfide clusters
of extreme biological significance should be mentioned. In
nature Fe–S clusters are found in proteins and enzymes.
Nitrogenases, for example, are Mo dependent Fe–S en-
zymes that catalytically participate in the six-electron re-
duction of elemental nitrogen to ammonia, a more bio-
logically useful form of nitrogen. Nitrogenases promote
many other reductive processes including the reduction of
acetylene to ethylene. The active site in these enzymes are
thought to be Fe–S cores or Mo–Fe–S cores. In the labo-
FIGURE 35 Structure of Co 6 (µ 6 -P)(CO) 16 . The open structure
of the Co 6 core is necessary to accommodate a µ 6 -P. (Terminal ratory many cluster compounds have been synthesized to
CO are not shown.) act as models of these enzymes.