Page 285 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 285
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
542 Metal Particles and Cluster Compounds
pair. Utilizing either of these electron sources brings the
net electron donation of the CO ligand to four. This type of
2
bonding should be indicated with a µ bonding descriptor.
2
A (η 2 , µ -CO) is observed in Mn 2 (CO) 5 -(Ph 2 PCH 2
PPh 2 ) 2 . The CO stretching frequency of 1645 cm −1 re-
sponds to the removal of π-bond density as well as π ∗
orbital population. The asymmetric attachment of CO re-
duces the overlap responsible for backbonding from the
Mn atom receiving the carbon lone pair sigma donation.
However, the side-on bonding of CO to the other Mn atom
offers another means by which backbonding can occur.
Figure 30 shows this side-on bonding of CO. This same
type of interaction is also responsible for the side-on bond-
ing of olefins to metal centers.
Another example of a four-electron-donating carbonyl
2
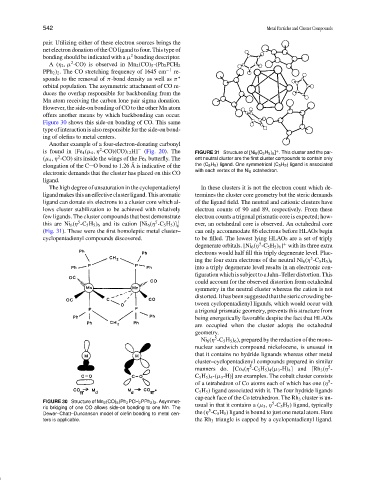
is found in [Fe 4 (µ 4 , η -CO)(CO) 12 H] − (Fig. 20). The FIGURE 31 Structure of [Ni 6 (C 5 H 5 ) 6 ] . This cluster and the par-
+
2
(µ 4 , η -CO) sits inside the wings of the Fe 4 butterfly. The ent neutral cluster are the first cluster compounds to contain only
˚
elongation of the C O bond to 1.26 A is indicative of the the (C 5 H 5 ) ligand. One symmetrical (C 5 H 5 ) ligand is associated
with each vertex of the Ni 6 octahedron.
electronic demands that the cluster has placed on this CO
ligand.
The high degree of unsaturation in the cyclopentadienyl In these clusters it is not the electron count which de-
ligand makes this an effective cluster ligand. This aromatic termines the cluster core geometry but the steric demands
ligand can donate six electrons to a cluster core which al- of the ligand field. The neutral and cationic clusters have
lows cluster stabilization to be achieved with relatively electron counts of 90 and 89, respectively. From these
few ligands. The cluster compounds that best demonstrate electron counts a trigonal prismatic core is expected; how-
5
5
this are Ni 6 (η -C 5 H 5 ) 6 and its cation [Ni 6 (η -C 5 H 5 )] + ever, an octahedral core is observed. An octahedral core
6
(Fig. 31). These were the first homoleptic metal cluster– can only accommodate 86 electrons before HLAOs begin
cyclopentadienyl compounds discovered. to be filled. The lowest lying HLAOs are a set of triply
5
degenerate orbitals. [Ni 6 (η -C 5 H 5 ) 6 ] with its three extra
+
electrons would half fill this triply degenerate level. Plac-
5
ing the four extra electrons of the neutral Ni 6 (η -C 5 H 5 ) 6
into a triply degenerate level results in an electronic con-
figuration which is subject to a Jahn–Teller distortion. This
could account for the observed distortion from octahedral
symmetry in the neutral cluster whereas the cation is not
distorted. It has been suggested that the steric crowding be-
tween cyclopentadienyl ligands, which would occur with
a trigonal prismatic geometry, prevents this structure from
being energetically favorable despite the fact that HLAOs
are occupied when the cluster adopts the octahedral
geometry.
5
Ni 6 (η -C 5 H 5 ) 6 ), prepared by the reduction of the mono-
nuclear sandwich compound nickelocene, is unusual in
that it contains no hydride lignands whereas other metal
cluster–cyclopentadienyl compounds prepared in similar
5
5
manners do. [Co 4 (η -C 5 H 5 ) 4 (µ 3 -H) 4 ] and [Rh 3 (η -
C 5 H 5 ) 4 -(µ 3 -H)] are examples. The cobalt cluster consists
5
of a tetrahedron of Co atoms each of which has one (η -
C 5 H 5 ) ligand associated with it. The four hydride ligands
cap each face of the Co tetrahedron. The Rh 3 cluster is un-
FIGURE 30 Structure of Mn 2 (CO) 5 (Ph 2 PCH 2 PPh 2 ) 2 . Asymmet- 5
ric bridging of one CO allows side-on bonding to one Mn. The usual in that it contains a (µ 3 ,η -C 5 H 5 ) ligand, typically
5
Dewar–Chatt–Duncanson model of olefin bonding to metal cen- the (η -C 5 H 5 ) ligand is bound to just one metal atom. Here
ters is applicable. the Rh 3 triangle is capped by a cyclopentadienyl ligand.