Page 282 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 282
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
Metal Particles and Cluster Compounds 539
Rh 6 (CO) 16 is an octahedral cluster with six Rh(CO) 2
vertices and four triply bridging carbonyls. Being a closo,
six-vertex cluster the PSEP theory would predict this
cluster to contain seven skeletal electron pairs. Each
Rh(CO) 2 vertex contributes one electron to the cluster
core and each (µ 3 -CO) contributes two electrons. The to-
tal skeletal electron count being 14 or 7 pairs as the theory
would predict.
There are numerous metal cluster-alkyne complexes
whichhavetheC Cunitofthealkynepositionedsuchthat
it occupies two vertices of a regular polyhedron (Fig. 27).
For example, there are many octahedral clusters with an
M 4 C 2 core. There is a significant distortion from pure oc-
tahedral symmetry due to the differing M M, M C, and
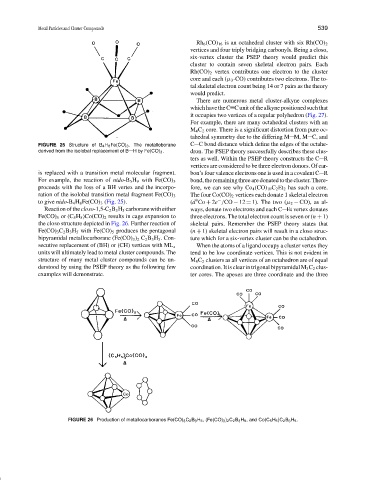
FIGURE 25 Structure of B 4 H 8 Fe(CO) 3 . The metalloborane C C bond distance which define the edges of the octahe-
derived from the isolobal replacement of B H by Fe(CO) 3 . dron. The PSEP theory successfully describes these clus-
ters as well. Within the PSEP theory constructs the C R
vertices are considered to be three electron donors. Of car-
is replaced with a transition metal molecular fragment. bon’s four valence electrons one is used in a covalent C R
bond, the remaining three are donated to the cluster. There-
For example, the reaction of nido-B 5 H 9 with Fe(CO) 5
proceeds with the loss of a BH vertex and the incorpo- fore, we can see why Co 4 (CO) 10 C 2 Et 2 has such a core.
ration of the isolobal transition metal fragment Fe(CO) 3 The four Co(CO) 2 vertices each donate 1 skeletal electron
9
to give nido-B 4 H 8 Fe(CO) 3 (Fig. 25). (d Co + 2e /CO − 12 = 1). The two (µ 2 − CO), as al-
−
Reaction of the closo-1,5-C 2 B 3 H 5 carborane with either ways, donate two electrons and each C Et vertex donates
Fe(CO) 5 or (C 5 H 5 )Co(CO) 2 results in cage expansion to three electrons. The total electron count is seven or (n + 1)
the closo structure depicted in Fig. 26. Further reaction of skeletal pairs. Remember the PSEP theory states that
Fe(CO) 3 C 2 B 3 H 5 with Fe(CO) 5 produces the pentagonal (n + 1) skeletal electron pairs will result in a closo struc-
bipyramidal metallocarborane (Fe(CO) 3 ) 2 C 2 B 3 H 5 . Con- ture which for a six-vertex cluster can be the octahedron.
secutive replacement of (BH) or (CH) vertices with ML x When the atoms of a ligand occupy a cluster vertex they
units will ultimately lead to metal cluster compounds. The tend to be low coordinate vertices. This is not evident in
structure of many metal cluster compounds can be un- M 4 C 2 clusters as all vertices of an octahedron are of equal
derstood by using the PSEP theory as the following few coordination. It is clear in trigonal bipyramidal M 3 C 2 clus-
examples will demonstrate. ter cores. The apexes are three coordinate and the three
FIGURE 26 Production of metallocarboranes Fe(CO) 3 C 2 B 3 H 5 , (Fe(CO) 3 ) 2 C 2 B 3 H 5 , and Co(C 5 H 5 )C 2 B 3 H 5 .