Page 279 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 279
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
536 Metal Particles and Cluster Compounds
bound. The Os to CO ratio of 5:16 precludes an even dis-
tribution of the sixteen carbonyl ligands. Four of the five
Os atoms have three terminally bound carbonyls. The four
remaining carbonyls are bound to the fifth Os atom. This
unique Os atom is positioned in an equatorial site of the
trigonal bipyramid. Because of the asymmetrical place-
ment of the CO ligands about the cluster there is a core
distortion from a pure trigonal bipyramid geometry.
Three possible geometries for a cluster containing six
metal atoms are the octahedron, the capped square pyra-
mid, and the bicapped tetrahedron which possess 43,
43, and 42 CVMOs, respectively. The 86 cluster va-
lence electrons of Co 6 (CO) 16 suggest the bicapped tetra-
hedron would be an unstable cluster configuration since
this would place two electrons in an HLAO. The observed
octahedral geometry with its 43 CVMOs can accommo-
date the 86 cluster valence electrons.
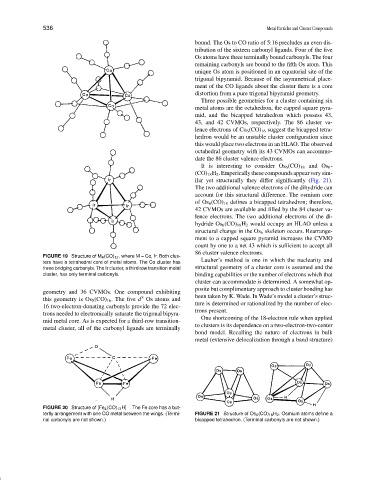
It is interesting to consider Os 6 (CO) 18 and Os 6 -
(CO) 18 H 2 .Empericallythesecompoundsappearverysim-
ilar yet structurally they differ significantly (Fig. 21).
The two additional valence electrons of the dihydride can
account for this structural difference. The osmium core
of Os 6 (CO) 18 defines a bicapped tetrahedron; therefore,
42 CVMOs are available and filled by the 84 cluster va-
lence electrons. The two additional electrons of the di-
hydride Os 6 (CO) 18 H 2 would occupy an HLAO unless a
structural change in the Os 6 skeleton occurs. Rearrange-
ment to a capped square pyramid increases the CVMO
count by one to a net 43 which is sufficient to accept all
86 cluster valence electrons.
FIGURE 19 Structure of M 4 (CO) 12 , where M = Co, Ir. Both clus-
ters have a tetrahedral core of metal atoms. The Co cluster has Lauher’s method is one in which the nuclearity and
three bridging carbonyls. The Ir cluster, a third row transition metal structural geometry of a cluster core is assumed and the
cluster, has only terminal carbonyls. binding capabilities or the number of electrons which that
cluster can accommodate is determined. A somewhat op-
posite but complimentary approach to cluster bonding has
geometry and 36 CVMOs. One compound exhibiting
8
this geometry is Os 5 (CO) 16 . The five d Os atoms and been taken by K. Wade. In Wade’s model a cluster’s struc-
ture is determined or rationalized by the number of elec-
16 two-electron-donating carbonyls provide the 72 elec-
trons present.
trons needed to electronically saturate the trigonal bipyra-
One shortcoming of the 18-electron rule when applied
mid metal core. As is expected for a third-row transition-
to clusters is its dependence on a two-electron-two-center
metal cluster, all of the carbonyl ligands are terminally
bond model. Recalling the nature of electrons in bulk
metal (extensive delocalization through a band structure)
FIGURE 20 Structure of [Fe 4 (CO) 13 H] . The Fe core has a but-
−
terfly arrangement with one CO metal between the wings. (Termi- FIGURE 21 Structure of Os 6 (CO) 18 H 2 . Osmium atoms define a
nal carbonyls are not shown.) bicapped tetrahedron. (Terminal carbonyls are not shown.)