Page 287 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 287
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
544 Metal Particles and Cluster Compounds
are relatively few examples known. Note in the examples,
shown below, that they are each µ 4 -donors thereby re-
taining their typical tetrahedral coordination. They are all
acting as surface µ 3 -donors to an M 3 triangle while being
capped by a fourth metal. The distance between this fourth
metal atom and the M 3 base precludes metal–metal bond-
ing so these atomic donor ligands would rightly be con-
sidered surface type donors rather than interstitials. This
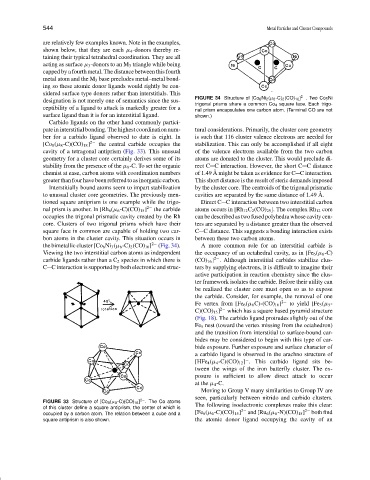
FIGURE 34 Structure of [Co 6 Ni 2 (µ 6 -C) 2 (CO) 16 ] 2− . Two Co 5 Ni
designation is not merely one of semantics since the sus-
trigonal prisms share a common Co 4 square face. Each trigo-
ceptibility of a ligand to attack is markedly greater for a nal prism encapsulates one carbon atom. (Terminal CO are not
surface ligand than it is for an interstitial ligand. shown.)
Carbido ligands on the other hand commonly partici-
pate in interstitial bonding. The highest coordination num- tural considerations. Primarily, the cluster core geometry
ber for a carbido ligand observed to date is eight. In is such that 116 cluster valence electrons are needed for
2−
[Co 8 (µ 8 -C)(CO) 18 ] the central carbide occupies the stabilization. This can only be accomplished if all eight
cavity of a tetragonal antiprism (Fig. 33). This unusual of the valence electrons available from the two carbon
geometry for a cluster core certainly derives some of its atoms are donated to the cluster. This would preclude di-
stability from the presence of the µ 8 -C. To set the organic rect C C interaction. However, the short C C distance
˚
chemist at ease, carbon atoms with coordination numbers of 1.49 A might be taken as evidence for C C interaction.
greater than four have been referred to as inorganic carbon. This short distance is the result of steric demands imposed
Interstitially bound atoms seem to impart stabilization by the cluster core. The centroids of the trigonal prismatic
˚
to unusual cluster core geometries. The previously men- cavities are separated by the same distance of 1.49 A.
tioned square antiprism is one example while the trigo- Direct C C interaction between two interstitial carbon
nal prism is another. In [Rh 6 (µ 6 -C)(CO) 15 ] 2− the carbide atoms occurs in [Rh 12 C 2 (CO) 25 ]. The complex Rh 12 core
occupies the trigonal prismatic cavity created by the Rh can be described as two fused polyhedra whose cavity cen-
core. Clusters of two trigonal prisms which have their ters are separated by a distance greater than the observed
square face in common are capable of holding two car- C C distance. This suggests a bonding interaction exists
bon atoms in the cluster cavity. This situation occurs in between these two carbon atoms.
the bimetallic cluster [Co 6 Ni 3 (µ 6 -C) 2 (CO) 16 ] 2− (Fig. 34). A more common role for an interstitial carbide is
Viewing the two interstitial carbon atoms as independent the occupancy of an octahedral cavity, as in [Fe 6 (µ 6 -C)
2−
carbide ligands rather than a C 2 species in which there is (CO) 16 ] . Although interstitial carbides stabilize clus-
C C interaction is supported by both electronic and struc- ters by supplying electrons, it is difficult to imagine their
active participation in reaction chemistry since the clus-
ter framework isolates the carbide. Before their utility can
be realized the cluster core must open so as to expose
the carbide. Consider, for example, the removal of one
Fe vertex from [Fe 6 (µ 6 C)-(CO) 16 ] 2− to yield [Fe 5 (µ 5 -
C)(CO) 15 ] 2− which has a square based pyramid structure
(Fig. 18). The carbido ligand protrudes slightly out of the
Fe 5 nest (toward the vertex missing from the octahedron)
and the transition from interstitial to surface-bound car-
bides may be considered to begin with this type of car-
bide exposure. Further exposure and surface character of
a carbido ligand is observed in the arachno structure of
[HFe 4 (µ 4 -C)(CO) 12 ] . This carbido ligand sits be-
−
tween the wings of the iron butterfly cluster. The ex-
posure is sufficient to allow direct attack to occur
at the µ 4 -C.
Moving to Group V many similarities to Group IV are
seen, particularly between nitrido and carbido clusters.
FIGURE 33 Structure of [Co 8 (µ 8 -C)(CO) 18 ] 2− . The Co atoms
of this cluster define a square antiprism, the center of which is The following isoelectronic complexes make this clear:
occupied by a carbon atom. The relation between a cube and a [Fe 6 (µ 6 -C)(CO) 15 ] 2− and [Ru 6 (µ 6 -N)(CO) 15 ] 2− both find
square antiprism is also shown. the atomic donor ligand occupying the cavity of an