Page 303 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 303
P1: GTV/GRI P2: GNH Final Pages
Encyclopedia of Physical Science and Technology EN010H-470 July 16, 2001 16:53
312 Nanosized Inorganic Clusters
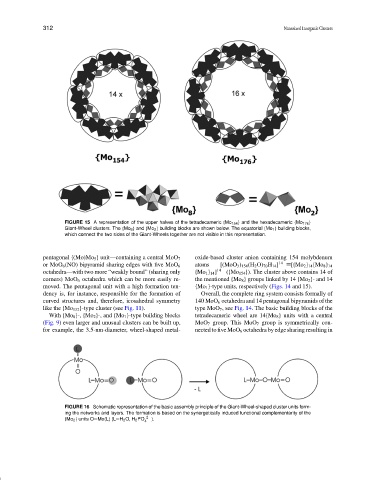
FIGURE 15 A representation of the upper halves of the tetradecameric {Mo 154 } and the hexadecameric {Mo 176 }
Giant-Wheel clusters. The {Mo 8 } and {Mo 2 } building blocks are shown below. The equatorial {Mo 1 } building blocks,
which connect the two sides of the Giant-Wheels together are not visible in this representation.
pentagonal {(Mo)Mo 5 } unit—containing a central MoO 7 oxide-based cluster anion containing 154 molybdenum
or MoO 6 (NO) bipyramid sharing edges with five MoO 6 atoms [(MoO 3 ) 154 (H 2 O) 70 H 14 ] 14− [{Mo 2 } 14 {Mo 8 } 14
octahedra—with two more “weakly bound” (sharing only {Mo 1 } 14 ] 14− ({Mo 154 }). The cluster above contains 14 of
corners) MoO 6 octahedra which can be more easily re- the mentioned {Mo 8 } groups linked by 14 {Mo 2 }- and 14
moved. The pentagonal unit with a high formation ten- {Mo 1 }-type units, respectively (Figs. 14 and 15).
dency is, for instance, responsible for the formation of Overall, the complete ring system consists formally of
curved structures and, therefore, icosahedral symmetry 140 MoO 6 octahedra and 14 pentagonal bipyramids of the
like the {Mo 132 }-type cluster (see Fig. 11). type MoO 7 , see Fig. 14. The basic building blocks of the
With {Mo 8 }-, {Mo 2 }-, and {Mo 1 }-type building blocks tetradecameric wheel are 14{Mo 8 } units with a central
(Fig. 9) even larger and unusual clusters can be built up, MoO 7 group. This MoO 7 group is symmetrically con-
for example, the 3.5-nm-diameter, wheel-shaped metal- nected to five MoO 6 octahedra by edge sharing resulting in
FIGURE 16 Schematic representation of the basic assembly principle of the Giant-Wheel-shaped cluster units form-
ing the networks and layers. The formation is based on the synergetically induced functional complementarity of the
{Mo 2 } units O=Mo(L) (L=H 2 O, H 2 PO 2− ).
4