Page 304 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 304
P1: GTV/GRI P2: GNH Final Pages
Encyclopedia of Physical Science and Technology EN010H-470 July 16, 2001 16:53
Nanosized Inorganic Clusters 313
an {(Mo)Mo 5 } pentagon. Four of the MoO 6 octahedra are with the formula [(MoO 3 ) 176 (H 2 O) 80 H 16 ] 16− comprises
linkedtotwoMoO 6 octahedraviacornerstoformthebasic two extra {Mo 8 }, {Mo 2 }, and {Mo 1 } units, respectively,
{Mo 8 } unit. Continuing from the {Mo 8 } unit the complete when compared to the smaller {Mo 154 } cluster, see Fig. 15.
{Mo 154 } cluster ring is built up as follows: (1) those two
MoO 6 octahedra which are not directly connected to the
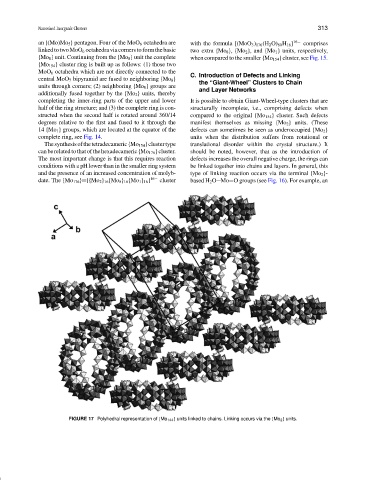
C. Introduction of Defects and Linking
central MoO 7 bipyramid are fused to neighboring {Mo 8 } the “Giant-Wheel” Clusters to Chain
units through corners; (2) neighboring {Mo 8 } groups are
and Layer Networks
additionally fused together by the {Mo 2 } units, thereby
completing the inner-ring parts of the upper and lower It is possible to obtain Giant-Wheel-type clusters that are
half of the ring structure; and (3) the complete ring is con- structurally incomplete, i.e., comprising defects when
structed when the second half is rotated around 360/14 compared to the original {Mo 154 } cluster. Such defects
degrees relative to the first and fused to it through the manifest themselves as missing {Mo 2 } units. (These
14 {Mo 1 } groups, which are located at the equator of the defects can sometimes be seen as underoccupied {Mo 2 }
complete ring, see Fig. 14. units when the distribution suffers from rotational or
Thesynthesisofthetetradecameric{Mo 154 }clustertype translational disorder within the crystal structure.) It
canberelatedtothatofthehexadecameric{Mo 176 }cluster. should be noted, however, that as the introduction of
The most important change is that this requires reaction defects increases the overall negative charge, the rings can
conditions with a pH lower than in the smaller ring system be linked together into chains and layers. In general, this
and the presence of an increased concentration of molyb- type of linking reaction occurs via the terminal {Mo 2 }-
date. The {Mo 176 } [{Mo 2 } 16 {Mo 8 } 16 {Mo 1 } 16 ] 16− cluster based H 2 O Mo=O groups (see Fig. 16). For example, an
FIGURE 17 Polyhedral representation of {Mo 144 } units linked to chains. Linking occurs via the {Mo 2 } units.