Page 311 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 311
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN010b-481 July 14, 2001 18:45
Noble Metals (Chemistry) 465
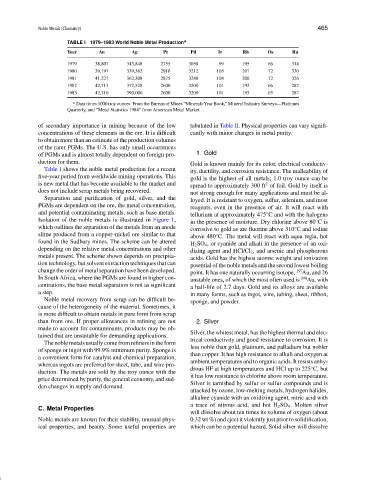
TABLE I 1979–1983 World Noble Metal Production a
Year Au Ag Pt Pd Ir Rh Os Ru
1979 38,807 343,848 2755 3058 99 195 66 314
1980 39,197 339,382 2910 3212 105 207 72 330
1981 41,227 362,308 2875 3248 108 206 72 326
1982 42,713 372,528 2600 3200 101 192 66 287
1983 42,710 390,000 2600 3200 101 193 65 287
a Data times 1000 troy ounces. From the Bureau of Mines “Minerals Year Book,” Mineral Industry Surveys—Platinum
Quarterly, and “Metal Statistics 1984” from American Metal Market.
of secondary importance in mining because of the low tabulated in Table II. Physical properties can vary signifi-
concentrations of these elements in the ore. It is difficult cantly with minor changes in metal purity.
to obtain more than an estimate of the production volumes
of the rarer PGMs. The U.S. has only small occurrences
of PGMs and is almost totally dependent on foreign pro- 1. Gold
duction for them. Gold is known mainly for its color, electrical conductiv-
Table I shows the noble metal production for a recent ity, ductility, and corrosion resistance. The malleability of
five-year period from worldwide mining operations. This gold is the highest of all metals; 1.0 troy ounce can be
is new metal that has become available to the market and spread to approximately 300 ft of foil. Gold by itself is
2
does not include scrap metals being recovered. not strong enough for many applications and must be al-
Separation and purification of gold, silver, and the loyed. It is resistant to oxygen, sulfur, selenium, and most
PGMs are dependent on the ore, the metal concentration, reagents, even in the presence of air. It will react with
and potential contaminating metals, such as base metals. tellurium at approximately 475 C and with the halogens
◦
Isolation of the noble metals is illustrated in Figure 1, in the presence of moisture. Dry chlorine above 80 Cis
◦
which outlines the separation of the metals from an anode corrosive to gold as are fluorine above 310 C and iodine
◦
slime produced from a copper–nickel ore similar to that above 480 C. The metal will react with aqua regia, hot
◦
found in the Sudbury mines. The scheme can be altered H 2 SO 4 , or cyanide and alkali in the presence of an oxi-
depending on the relative metal concentrations and other dizing agent and HCl/Cl 2 , and arsenic and phosphorous
metals present. The scheme shown depends on precipita- acids. Gold has the highest atomic weight and ionization
tion technology, but solvent extraction techniques that can
potential of the noble metals and the second lowest boiling
change the order of metal separation have been developed. 197
point. It has one naturally occurring isotope, Au, and 26
In South Africa, where the PGMs are found in higher con- 198
unstable ones, of which the most often used is Au, with
centrations, the base metal separation is not as significant
a half-life of 2.7 days. Gold and its alloys are available
a step.
in many forms, such as ingot, wire, tubing, sheet, ribbon,
Noble metal recovery from scrap can be difficult be-
sponge, and powder.
cause of the heterogeneity of the material. Sometimes, it
is more difficult to obtain metals in pure form from scrap
than from ore. If proper allowances in refining are not 2. Silver
made to account for contaminants, products may be ob-
Silver, the whitest metal, has the highest thermal and elec-
tained that are unsuitable for demanding applications.
trical conductivity and good resistance to corrosion. It is
The noble metals usually come from refiners in the form
less noble than gold, platinum, and palladium but nobler
of sponge or ingot with 99.9% minimum purity. Sponge is
than copper. It has high resistance to alkali and oxygen at
a convenient form for catalyst and chemical preparation,
ambient temperatures and to organic acids. It resists anhy-
whereas ingots are preferred for sheet, tube, and wire pro-
drous HF at high temperatures and HCl up to 225 C, but
◦
duction. The metals are sold by the troy ounce with the
it has low resistance to chlorine above room temperature.
price determined by purity, the general economy, and sud-
Silver is tarnished by sulfur or sulfur compounds and is
den changes in supply and demand.
attacked by ozone, low-melting metals, hydrogen halides,
alkaline cyanide with an oxidizing agent, nitric acid with
a trace of nitrous acid, and hot H 2 SO 4 . Molten silver
C. Metal Properties
will dissolve about ten times its volume of oxygen (about
Noble metals are known for their stability, unusual phys- 0.32 wt %) and eject it violently just prior to solidification,
ical properties, and beauty. Some useful properties are which can be a potential hazard. Solid silver will dissolve