Page 314 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 314
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN010b-481 July 14, 2001 18:45
468 Noble Metals (Chemistry)
a
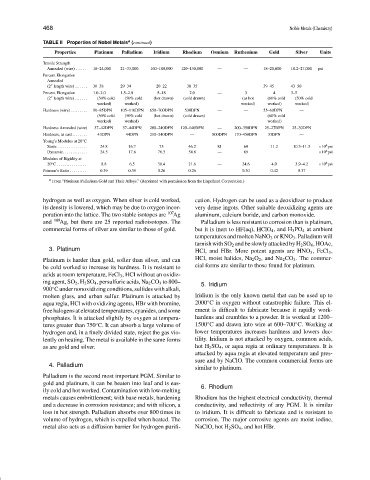
TABLE II Properties of Nobel Metals (continued)
Properties Platinum Palladium Iridium Rhodium Osmium Ruthenium Gold Silver Units
Tensile Strength
Annealed (wire) ...... 18–24,000 21–33,000 160–180,000 120–130,000 — — 18–20,000 18.2–27,000 psi
Percent Elongation
Annealed
(2 length wire)....... 30–38 29–34 20–22 30–35 — — 39–45 43–50
Percent Elongation 1.0–2.0 1.5–2.0 5–18 2.0 — 3 4 3–5
(2 length wire)....... (50% cold (50% cold (hot drawn) (cold drawn) (as hot (60% cold (50% cold
worked) worked) worked) worked) worked)
Hardness (wire)......... 90–95DPN 105–110DPN 650–700DPN 530DPN — — 55–60DPN —
(50% cold (50% cold (hot drawn) (cold drawn) (60% cold
worked) worked) worked)
Hardness Annealed (wire) 37–42DPN 37–44DPN 200–240DPN 120–140DPN — 200–350DPN 25–27DPN 25–30DPN
Hardness, as cast........ 43DPN 44DPN 210–240DPN — 800DPN 170–450DPN 33DPN —
◦
Young’s Modulus at 20 C
4
Static ................ 24.8 16.7 75 46.2 81 60 11.2 10.3–11.3 ×10 psi
4
Dynamic............. 24.5 17.6 76.5 54.8 — 69 ×10 psi
Modulus of Rigidity at
4
20 C ................ 8.8 6.5 30.4 21.6 — 24.6 4.0 3.9–4.2 ×10 psi
◦
Poisson’s Ratio ......... 0.39 0.39 0.26 0.26 — 0.30 0.42 0.37
a
From “Platinum Palladium Gold and Their Alloys.” (Reprinted with permission from the Engelhard Corporation.)
hydrogen as well as oxygen. When silver is cold worked, cation. Hydrogen can be used as a deoxidizer to produce
its density is lowered, which may be due to oxygen incor- very dense ingots. Other suitable deoxidizing agents are
poration into the lattice. The two stable isotopes are 107 Ag aluminum, calcium boride, and carbon monoxide.
and 109 Ag, but there are 25 reported radioisotopes. The Palladium is less resistant to corrosion than is platinum,
commercial forms of silver are similar to those of gold. but it is inert to HF(aq), HClO 4 , and H 3 PO 4 at ambient
temperatures and molten NaNO 3 or KNO 3 . Palladium will
tarnish with SO 2 and be slowly attacked by H 2 SO 4 , HOAc,
3. Platinum HCl, and HBr. More potent agents are HNO 3 , FeCl 3 ,
Platinum is harder than gold, softer than silver, and can HCl, moist halides, Na 2 O 2 , and Na 2 CO 3 . The commer-
be cold worked to increase its hardness. It is resistant to cial forms are similar to those found for platinum.
acids at room temperature, FeCl 3 , HCl without an oxidiz-
ing agent, SO 2 ,H 2 SO 4 , persulfuric acids, Na 2 CO 3 to 800–
5. Iridium
900 C under nonoxidizing conditions, sulfides with alkali,
◦
molten glass, and urban sulfur. Platinum is attacked by Iridium is the only known metal that can be used up to
◦
aqua regia, HCl with oxidizing agents, HBr with bromine, 2000 C in oxygen without catastrophic failure. This el-
freehalogensatelevatedtemperatures,cyanides,andsome ement is difficult to fabricate because it rapidly work-
phosphates. It is attacked slightly by oxygen at tempera- hardens and crumbles to a powder. It is worked at 1200–
◦
◦
tures greater than 750 C. It can absorb a large volume of 1500 C and drawn into wire at 600–700 C. Working at
◦
hydrogen and, in a finely divided state, reject the gas vio- lower temperatures increases hardness and lowers duc-
lently on heating. The metal is available in the same forms tility. Iridium is not attacked by oxygen, common acids,
as are gold and silver. hot H 2 SO 4 , or aqua regia at ordinary temperatures. It is
attacked by aqua regia at elevated temperature and pres-
sure and by NaClO. The common commercial forms are
4. Palladium
similar to platinum.
Palladium is the second most important PGM. Similar to
gold and platinum, it can be beaten into leaf and is eas-
6. Rhodium
ily cold and hot worked. Contamination with low-melting
metals causes embrittlement; with base metals, hardening Rhodium has the highest electrical conductivity, thermal
and a decrease in corrosion resistance; and with silicon, a conductivity, and reflectivity of any PGM. It is similar
loss in hot strength. Palladium absorbs over 800 times its to iridium. It is difficult to fabricate and is resistant to
volume of hydrogen, which is expelled when heated. The corrosion. The major corrosive agents are moist iodine,
metal also acts as a diffusion barrier for hydrogen purifi- NaClO, hot H 2 SO 4 , and hot HBr.