Page 317 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 317
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN010b-481 July 14, 2001 18:45
Noble Metals (Chemistry) 471
the second-row metals and their compounds are more re- the halides or pseudohalides and phosphorus, arsenic, sul-
active than their third-row counterparts. This introduction fur, and nitrogen-containing ligands. Gold(I) is a “soft”
to noble metal chemistry deals mainly with the diversity metal center with a preference for “soft” donor atoms.
of compounds and their preparations. A complete list of Substitution reactions follow this pattern. Organometal-
the common compounds is not given here because there lic derivatives include alkyl, aryl, alkenyl, alkynyl, and
are several excellent compilations. carbene complexes. They are usually formed by ligand
Thepreciousmetalsformavarietyofsalts,coordination exchange using a Grignard or organolithium reagent with
complexes, and organometallic compounds. The metals a gold(I) halide. Gold(I) π-complexes with olefins are not
are discussed individually to gain some understanding of as stable as the platinum analogs. The Au–C bond can be
how the compounds are formed and what ligands will stabilized with PR 3 , AsR 3 ,R 2 S, and RNC. Alkylgold(I)
stabilize a particular oxidation state. complexes can undergo oxidative addition with CH 3 lor
halogen to yield four-coordinate gold(III) derivatives.
1. Gold
Na[Au(CN) 2 ] and K[Au(CN) 2 ] are used extensively for
Gold compounds are known in the (−I), (I), (II), (III), and electrochemical or electroless guilding and in the recovery
(V) oxidation states. The linearly two-coordinate gold(I) and recycling of gold. Gold(I) thiosulfate, thiomalate, and
and square planar four-coordinate gold(III) complexes are thiogluconate esters are important gold drugs (chrysother-
the most common. apyofpolyarthritis).Goldthiolatesarethebasisfor“liquid
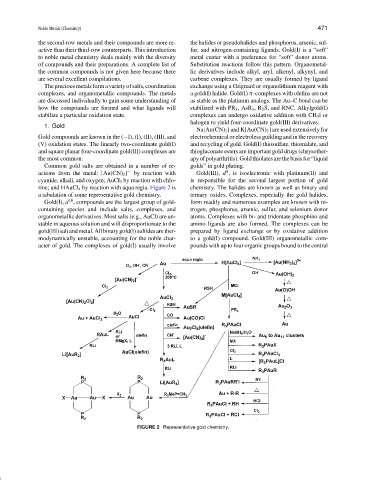
Common gold salts are obtained in a number of re- golds” in gold plating.
8
actions from the metal: [Au(CN) 2 ] − by reaction with Gold(III), d , is isoelectronic with platinum(II) and
cyanide, alkali, and oxygen; AuCl 3 by reaction with chlo- is responsible for the second largest portion of gold
rine; and HAuCl 4 by reaction with aqua regia. Figure 2 is chemistry. The halides are known as well as binary and
a tabulation of some representative gold chemistry. ternary oxides. Complexes, especially the gold halides,
10
Gold(I), d , compounds are the largest group of gold- form readily and numerous examples are known with ni-
containing species and include salts, complexes, and trogen, phosphorus, arsenic, sulfur, and selenium donor
organometallic derivatives. Most salts (e.g., AuCl) are un- atoms. Complexes with bi- and tridentate phosphino and
stable in aqueous solution and will disproportionate to the amino ligands are also formed. The complexes can be
gold(III)saltandmetal.Allbinarygold(I)sulfidesarether- prepared by ligand exchange or by oxidative addition
modynamically unstable, accounting for the noble char- to a gold(I) compound. Gold(III) organometallic com-
acter of gold. The complexes of gold(I) usually involve pounds with up to four organic groups bound to the central
FIGURE 2 Representative gold chemistry.