Page 318 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 318
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN010b-481 July 14, 2001 18:45
472 Noble Metals (Chemistry)
atom are available. Reductive elimination reactions lead phosphine, arsine, sulfide, and selenide ligand systems.
to organogold(I) compounds. Coordination complexes involving oxygen donor lig-
Gold(−I) is formed when the metal is reacted with ele- ands are less common. If excess ligand is present, small
mental cesium or with sodium or lithium in liquid amonia amounts of three- and four-coordinate products will form.
9
to yield CsAu, NaAu, or LiAu. Gold(II), d , is present in Complicatedpolynuclearsystemsresultwhenpolydentate
dinuclear gold compounds with direct Au–Au bonding, ligands are used, because of their inability to bond in a lin-
mainly in metalla-bicyclic prototypes, which are formed ear fashion around a single metal center. The organometal-
byoxidativeadditionofhalogenacrosstherings.Mononu- lic chemistry of silver(I) is very limited, with unstable
clear gold(II) species are found in dithiolene, dithiolate, olefin compounds making up the major fraction. Silver
alkyl and aryl species are much less stable than both the
and dicarbollyl complexes. Phosphorus ylides R 3 P = CH 2
form a particularly large class of stable organogold com- copper and the gold analogs. Phosphorus ylides form the
pounds (Fig. 2) of gold(I), gold(II) and gold(III). most stable organosilver(I) compounds (Fig. 3). With F 3 C
6
−
Gold(V), d , is known in AuF 5 and in [AuF 6 ] salts substituents, even organosilver(III) complexes can be iso-
that are prepared by the action of fluorine on the metal. lated. The olefin complexes can be obtained by direct re-
action with a silver(I) salt.
2. Silver 9
Silver(II), d , is paramagnetic and is present in salts
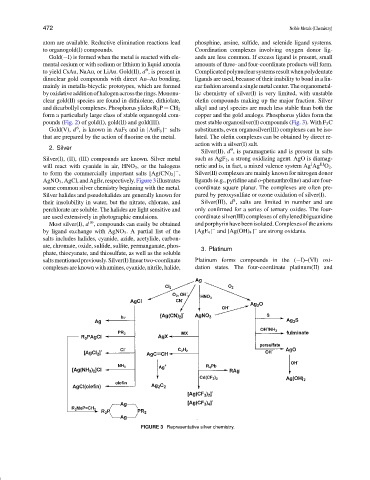
Silver(I), (II), (III) compounds are known. Silver metal such as AgF 2 , a strong oxidizing agent. AgO is diamag-
III
I
will react with cyanide in air, HNO 3 , or the halogens netic and is, in fact, a mixed valence system Ag Ag O 2 .
to form the commercially important salts [Ag(CN) 2 ] , Silver(II) complexes are mainly known for nitrogen donor
−
AgNO 3 , AgCl, and AgBr, respectively. Figure 3 illustrates ligands (e.g., pyridine and o-phenanthroline) and are four-
some common silver chemistry beginning with the metal. coordinate square planar. The complexes are often pre-
Silver halides and pseudohalides are generally known for pared by peroxysulfate or ozone oxidation of silver(I).
8
their insolubility in water, but the nitrate, chlorate, and Silver(III), d , salts are limited in number and are
perchlorate are soluble. The halides are light sensitive and only confirmed for a series of ternary oxides. The four-
are used extensively in photographic emulsions. coordinate silver(III) complexes of ethylenedibiguanidine
10
Most silver(I), d , compounds can easily be obtained andporphyrinhavebeenisolated.Complexesoftheanions
−
−
by ligand exchange with AgNO 3 . A partial list of the [AgF 4 ] and [Ag(OH) 4 ] are strong oxidants.
salts includes halides, cyanide, azide, acetylide, carbon-
ate, chromate, oxide, sulfide, sulfite, permanganate, phos-
3. Platinum
phate, thiocyanate, and thiosulfate, as well as the soluble
saltsmentionedpreviously.Silver(I)lineartwo-coordinate Platinum forms compounds in the (−I)–(VI) oxi-
complexes are known with amines, cyanide, nitrile, halide, dation states. The four-coordinate platinum(II) and
FIGURE 3 Representative silver chemistry.