Page 321 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 321
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN010b-481 July 14, 2001 18:45
Noble Metals (Chemistry) 475
arsine donor ligands. The complexes are formed by re- chemistry, with many of the reactions also applying to irid-
duction of iridium(IV) species or by oxidative addition ium. Rhodium is found in oxidation states (−I) through
to iridium(I) compounds. The organometallics comprise (V). The most common states are (I) and (III), with coor-
carbonyl, alkyl, aryl, and π-complexing ligands. The car- dination numbers of four (square planar) and six (octahe-
bonyls can be prepared by direct reaction of CO with the dral), respectively.
iridium(III) halide salt. The alkyl and aryl complexes are The metal is inert to aqua regia but Rh(OH) 3 can be pre-
synthesized using Grignard or lithium alkyl reagents. pared by fusing rhodium with sodium bisulfate followed
5
Iridium(IV), d , salts of halides, hydroxide, sulfide, se- by water and alkali. Rhodium(0) complexes are derived
lenide, and telluride have been reported. The commercial from RhCl 3 by direct reaction with CO to form Rh 2 (CO) 8 ,
product H 2 [IrCl 6 ] · 6H 2 O is water soluble and acts as a and the clusters Rh 4 (CO) 12 and Rh 6 (CO) 16 .
8
catalyst. IrO 2 is prepared from the hexahydroxo complex. Rhodium(I), d , compounds are comprised almost
Iridium(IV) does not form many complexes, but the six- entirely of π-acceptor ligand complexes, which serve to
coordinate halides (excluding the iodo species), and some stabilize the low oxidation state. The most significant lig-
amine and pyridine complexes are known. Compounds ands are carbonyl, phosphine, arsine, stibine, π-acceptor
with phosphorus, arsenic, and sulfur donor atoms are not nitrogen compounds, nitrosyl, cyanide, cyclopentadienyl,
stable; these ligands reduce iridium(IV) to the iridium(III) olefin, diene, acetylene, and allyl species. Complexes con-
state. taining hydride, halide, and oxygen donor ligands (e.g.,
3
4
Iridium(V), d , and iridium(VI), d , compounds are acetate and acetylacetonate) are known in combination
limited to the fluoride and oxide derivatives. Known with π-acceptor ligands. Rhodium(I) complexes are pre-
species are IrF 5 and [IrF 6 ] for iridium(V), and IrF 6 and pared by ligand exchange with another rhodium(I) com-
−
IrO 3 for iridium(VI). plex or by reducing a rhodium(III) species with alcohol,
SnCl 2 ,orCO · [(CO) 2 Rh(acac)], [(CO)Ph 3 P)Rh(acac)],
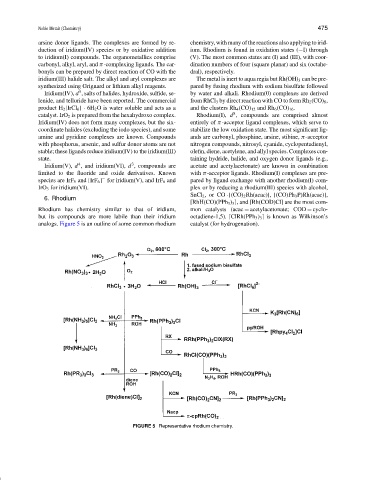
6. Rhodium
[RhH(CO)(PPh 3 ) 3 ], and [Rh(COD)Cl] are the most com-
Rhodium has chemistry similar to that of iridium, mon catalysts (acac = acetylacetonate; COD = cyclo-
but its compounds are more labile than their iridium octadiene-1,5). [ClRh(PPh 3 ) 3 ] is known as Wilkinson’s
analogs. Figure 5 is an outline of some common rhodium catalyst (for hydrogenation).
FIGURE 5 Representative rhodium chemistry.