Page 363 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 363
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
682 Periodic Table (Chemistry)
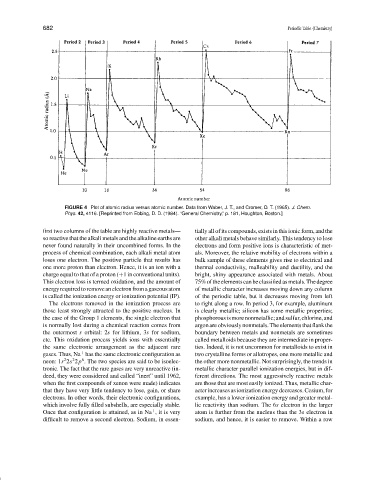
FIGURE 4 Plot of atomic radius versus atomic number. Data from Waber, J. T., and Cromer, D. T. (1965). J. Chem.
Phys. 42, 4116. [Reprinted from Ebbing, D. D. (1984). “General Chemistry,” p. 181, Houghton, Boston.]
first two columns of the table are highly reactive metals— tially all of its compounds, exists in this ionic form, and the
so reactive that the alkali metals and the alkaline earths are other alkali metals behave similarly. This tendency to lose
never found naturally in their uncombined forms. In the electrons and form positive ions is characteristic of met-
process of chemical combination, each alkali metal atom als. Moreover, the relative mobility of electrons within a
loses one electron. The positive particle that results has bulk sample of these elements gives rise to electrical and
one more proton than electron. Hence, it is an ion with a thermal conductivity, malleability and ductility, and the
charge equal to that of a proton (+1 in conventional units). bright, shiny appearance associated with metals. About
This electron loss is termed oxidation, and the amount of 75%oftheelementscanbeclassifiedasmetals.Thedegree
energyrequiredtoremoveanelectronfromagaseousatom of metallic character increases moving down any column
is called the ionization energy or ionization potential (IP). of the periodic table, but it decreases moving from left
The electrons removed in the ionization process are to right along a row. In period 3, for example, aluminum
those least strongly attracted to the positive nucleus. In is clearly metallic; silicon has some metallic properties;
the case of the Group 1 elements, the single electron that phosphorousismorenonmetallic;andsulfur,chlorine,and
is normally lost during a chemical reaction comes from argonareobviouslynonmetals.Theelementsthatflankthe
the outermost s orbital: 2s for lithium, 3s for sodium, boundary between metals and nonmetals are sometimes
etc. This oxidation process yields ions with essentially called metalloids because they are intermediate in proper-
the same electronic arrangement as the adjacent rare ties. Indeed, it is not uncommon for metalloids to exist in
gases. Thus, Na has the same electronic configuration as two crystalline forms or allotropes, one more metallic and
+
6
2
2
neon: 1s 2s 2p . The two species are said to be isoelec- the other more nonmetallic. Not surprisingly, the trends in
tronic. The fact that the rare gases are very unreactive (in- metallic character parallel ionization energies, but in dif-
deed, they were considered and called “inert” until 1962, ferent directions. The most aggressively reactive metals
when the first compounds of xenon were made) indicates are those that are most easily ionized. Thus, metallic char-
that they have very little tendency to lose, gain, or share acter increases as ionization energy decreases. Cesium, for
electrons. In other words, their electronic configurations, example, has a lower ionization energy and greater metal-
which involve fully filled subshells, are especially stable. lic reactivity than sodium. The 6s electron in the larger
Once that configuration is attained, as in Na ,itisvery atom is further from the nucleus than the 3s electron in
+
difficult to remove a second electron. Sodium, in essen- sodium, and hence, it is easier to remove. Within a row