Page 365 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 365
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
684 Periodic Table (Chemistry)
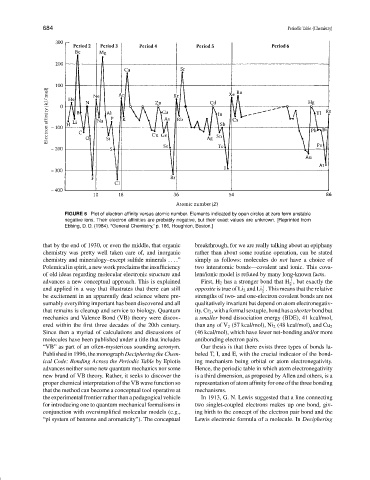
FIGURE 6 Plot of electron affinity versus atomic number. Elements indicated by open circles at zero form unstable
negative ions. Their electron affinities are probably negative, but their exact values are unknown. [Reprinted from
Ebbing, D. D. (1984). “General Chemistry,” p. 186, Houghton, Boston.]
that by the end of 1930, or even the middle, that organic breakthrough, for we are really talking about an epiphany
chemistry was pretty well taken care of, and inorganic rather than about some routine operation, can be stated
chemistry and mineralogy–except sulfide minerals ....” simply as follows: molecules do not have a choice of
Polemical in spirit, a new work proclaims the insufficiency two interatomic bonds—covalent and ionic. This cova-
of old ideas regarding molecular electronic structure and lent/ionic model is refuted by many long-known facts.
+
advances a new conceptual approach. This is explained First, H 2 has a stronger bond that H , but exactly the
2
+
and applied in a way that illustrates that there can still opposite is true of Li 2 and Li . This means that the relative
2
be excitement in an apparently dead science where pre- strengths of two- and one-electron covalent bonds are not
sumably everything important has been discovered and all qualitatively invariant but depend on atom electronegativ-
that remains is cleanup and service to biology. Quantum ity.Cr 2 ,withaformalsextuple,bondhasashorterbondbut
mechanics and Valence Bond (VB) theory were discov- a smaller bond dissociation energy (BDE), 41 kcal/mol,
ered within the first three decades of the 20th century. than any of V 2 (57 kcal/mol), Ni 2 (48 kcal/mol), and Cu 2
Since then a myriad of calculations and discussions of (46 kcal/mol), which have fewer net-bonding and/or more
molecules have been published under a title that includes antibonding electron pairs.
“VB” as part of an often-mysterious sounding acronym. Our thesis is that there exists three types of bonds la-
Published in 1996, the monograph Deciphering the Chem- beled T, I, and E, with the crucial indicator of the bond-
ical Code: Bonding Across the Periodic Table by Epiotis ing mechanism being orbital or atom electronegativity.
advances neither some new quantum mechanics nor some Hence, the periodic table in which atom electronegativity
new brand of VB theory. Rather, it seeks to discover the is a third dimension, as proposed by Allen and others, is a
proper chemical interpretation of the VB wave function so representation of atom affinity for one of the three bonding
that the method can become a conceptual tool operative at mechanisms.
the experimental frontier rather than a pedagogical vehicle In 1913, G. N. Lewis suggested that a line connecting
for introducing one to quantum mechanical formalisms in two singlet-coupled electrons makes up one bond, giv-
conjunction with oversimplified molecular models (e.g., ing birth to the concept of the electron pair bond and the
“pi system of benzene and aromaticity”). The conceptual Lewis electronic formula of a molecule. In Deciphering