Page 370 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 370
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
Periodic Table (Chemistry) 689
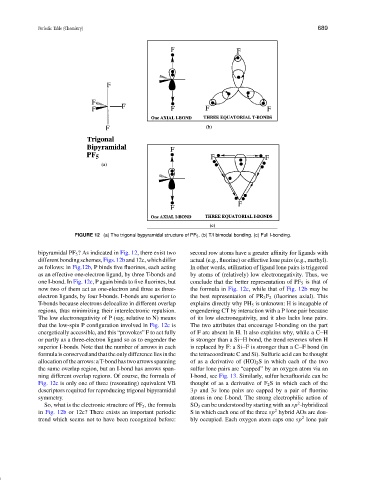
FIGURE 12 (a) The trigonal bypyramidal structure of PF 5 . (b) T/I bimodal bonding. (c) Full I-bonding.
bipyramidal PF 5 ? As indicated in Fig. 12, there exist two second row atoms have a greater affinity for ligands with
different bonding schemes, Figs. 12b and 12c, which differ actual (e.g., fluorine) or effective lone pairs (e.g., methyl).
as follows: in Fig.12b, P binds five fluorines, each acting In other words, utilization of ligand lone pairs is triggered
as an effective one-electron ligand, by three T-bonds and by atoms of (relatively) low electronegativity. Thus, we
one I-bond. In Fig. 12c, P again binds to five fluorines, but conclude that the better representation of PF 5 is that of
now two of them act as one-electron and three as three- the formula in Fig. 12c, while that of Fig. 12b may be
electron ligands, by four I-bonds. I-bonds are superior to the best representation of PR 3 F 2 (fluorines axial). This
T-bonds because electrons delocalize in different overlap explains directly why PH 5 is unknown: H is incapable of
regions, thus minimizing their interelectronic repulsion. engendering CT by interaction with a P lone pair because
The low electronegativity of P (say, relative to N) means of its low electronegativity, and it also lacks lone pairs.
that the low-spin P configuration involved in Fig. 12c is The two attributes that encourage I-bonding on the part
energetically accessible, and this “provokes” F to act fully of F are absent in H. It also explains why, while a C H
or partly as a three-electron ligand so as to engender the is stronger than a Si H bond, the trend reverses when H
superior I-bonds. Note that the number of arrows in each is replaced by F: a Si F is stronger than a C F bond (in
formula is conserved and that the only difference lies in the the tetracoordinate C and Si). Sulfuric acid can be thought
allocation of the arrows: a T-bond has two arrows spanning of as a derivative of (HO) 2 S in which each of the two
the same overlap region, but an I-bond has arrows span- sulfur lone pairs are “capped” by an oxygen atom via an
ning different overlap regions. Of course, the formula of I-bond, see Fig. 13. Similarly, sulfur hexafluoride can be
Fig. 12c is only one of three (resonating) equivalent VB thought of as a derivative of F 2 S in which each of the
descriptors required for reproducing trigonal bipyramidal 3p and 3s lone pairs are capped by a pair of fluorine
symmetry. atoms in one I-bond. The strong electrophilic action of
2
So, what is the electronic structure of PF 5 , the formula SO 3 can be understood by starting with an sp -hybridized
2
in Fig. 12b or 12c? There exists an important periodic S in which each one of the three sp hybrid AOs are dou-
2
trend which seems not to have been recognized before: bly occupied. Each oxygen atom caps one sp lone pair