Page 371 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 371
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
690 Periodic Table (Chemistry)
FIGURE 13 The capping of one lone pair of S by an oxygen atom
via one I-bond. The two representations are essentially equivalent,
differing only in the selection of the reference configuration.
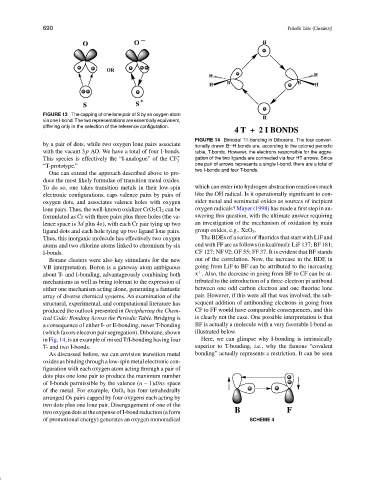
FIGURE 14 Bimodal T/I-bonding in Diborane. The four conven-
by a pair of dots, while two oxygen lone pairs associate tionally drawn B H bonds are, according to the colored periodic
with the vacant 3p AO. We have a total of four I-bonds. table, T-bonds. However, the electrons responsible for the aggre-
This species is effectively the “I-analogue” of the CF + gation of the two ligands are connected via four HT arrows. Since
3
“T-prototype.” one pair of arrows represents a single I-bond, there are a total of
two I-bonds and four T-bonds.
One can extend the approach described above to pro-
duce the most likely formulae of transition metal oxides.
To do so, one takes transition metals in their low-spin which can enter into hydrogen abstraction reactions much
electronic configurations, caps valence pairs by pairs of like the OH radical. Is it operationally significant to con-
oxygen dots, and associates valence holes with oxygen sider metal and semimetal oxides as sources of incipient
lone pairs. Thus, the well-known oxidizer CrO 2 Cl 2 can be oxygen radicals? Mayer (1998) has made a first step in an-
formulated as Cr with three pairs plus three holes (the va- swering this question, with the ultimate answer requiring
lence space is 3d plus 4s), with each Cr pair tying up two an investigation of the mechanism of oxidation by main
ligand dots and each hole tying up two ligand lone pairs. group oxides, e.g., XeO 3 .
Thus, this inorganic molecule has effectively two oxygen The BDEs of a series of fluorides that start with LiF and
atoms and two chlorine atoms linked to chromium by six end with FF are as follows (in kcal/mol): LiF 137; BF 181;
I-bonds. CF 127; NF 92; OF 55; FF 37. It is evident that BF stands
Borane clusters were also key stimulants for the new out of the correlation. Now, the increase in the BDE in
VB interpretation. Boron is a gateway atom ambiguous going from LiF to BF can be attributed to the increasing
+
about T- and I-bonding, advantageously combining both x . Also, the decrease in going from BF to CF can be at-
mechanisms as well as being tolerant to the expression of tributed to the introduction of a three-electron pi antibond
either one mechanism acting alone, generating a fantastic between one odd carbon electron and one fluorine lone
array of diverse chemical systems. An examination of the pair. However, if this were all that was involved, the sub-
structural, experimental, and computational literature has sequent addition of antibonding electrons in going from
produced the outlook presented in Deciphering the Chem- CF to FF would have comparable consequences, and this
ical Code: Bonding Across the Periodic Table. Bridging is is clearly not the case. One possible interpretation is that
a consequence of either I- or E-bonding, never T-bonding BF is actually a molecule with a very favorable I-bond as
(which favors electron pair segregation). Diborane, shown illustrated below.
in Fig. 14, is an example of mixed T/I-bonding having four Here, we can glimpse why I-bonding is intrinsically
T- and two I-bonds. superior to T-bonding, i.e., why the famous “covalent
As discussed before, we can envision transition metal bonding” actually represents a restriction. It can be seen
oxides as binding through a low-spin metal electronic con-
figuration with each oxygen atom acting through a pair of
dots plus one lone pair to produce the maximum number
of I-bonds permissible by the valence (n − 1)d/ns space
of the metal. For example, OsO 4 has four tetrahedrally
arranged Os pairs capped by four oxygens each acting by
two dots plus one lone pair. Disengagement of one of the
two oxygen dots at the expense of I-bond reduction (a form
of promotional energy) generates an oxygen monoradical SCHEME 4