Page 373 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 373
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
692 Periodic Table (Chemistry)
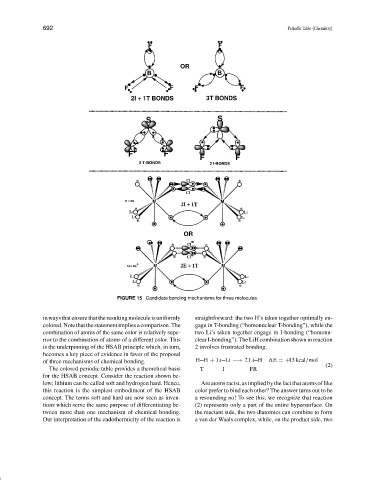
FIGURE 15 Candidate bonding mechanisms for three molecules.
inwaysthatensurethattheresultingmoleculeisuniformly straightforward: the two H’s taken together optimally en-
colored.Notethatthestatementimpliesacomparison.The gage in T-bonding (“homonuclear T-bonding”), while the
combination of atoms of the same color is relatively supe- two Li’s taken together engage in I-bonding (“homonu-
rior to the combination of atoms of a different color. This clear I-bonding”). The LiH combination shown in reaction
is the underpinning of the HSAB principle which, in turn, 2 involves frustrated bonding.
becomes a key piece of evidence in favor of the proposal
of three mechanisms of chemical bonding. H H + Li Li −→ 2Li H
E =+13 kcal/mol
(2)
The colored periodic table provides a theoretical basis T I FR
for the HSAB concept. Consider the reaction shown be-
low; lithium can be called soft and hydrogen hard. Hence, Are atoms racist, as implied by the fact that atoms of like
this reaction is the simplest embodiment of the HSAB color prefer to bind each other? The answer turns out to be
concept. The terms soft and hard are now seen as inven- a resounding no! To see this, we recognize that reaction
tions which serve the same purpose of differentiating be- (2) represents only a part of the entire hypersurface. On
tween more than one mechanism of chemical bonding. the reactant side, the two diatomics can combine to form
Our interpretation of the endothermicity of the reaction is a van der Waals complex, while, on the product side, two