Page 364 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 364
P1: GTQ/GUU P2: GTQ Final Pages
Encyclopedia of Physical Science and Technology EN011H-551 July 25, 2001 18:33
Periodic Table (Chemistry) 683
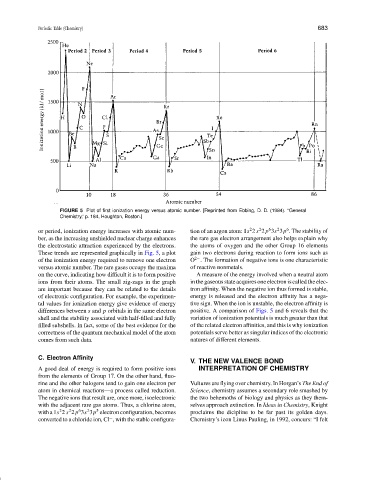
FIGURE 5 Plot of first ionization energy versus atomic number. [Reprinted from Ebbing, D. D. (1984). “General
Chemistry,” p. 184, Houghton, Boston.]
6
6
2
2
2
or period, ionization energy increases with atomic num- tion of an argon atom: 1s 2 s 2 p 3s 3p . The stability of
ber, as the increasing unshielded nuclear charge enhances the rare gas electron arrangement also helps explain why
the electrostatic attraction experienced by the electrons. the atoms of oxygen and the other Group 16 elements
These trends are represented graphically in Fig. 5, a plot gain two electrons during reaction to form ions such as
of the ionization energy required to remove one electron O . The formation of negative ions is one characteristic
2−
versus atomic number. The rare gases occupy the maxima of reactive nonmetals.
on the curve, indicating how difficult it is to form positive A measure of the energy involved when a neutral atom
ions from their atoms. The small zig-zags in the graph in the gaseous state acquires one electron is called the elec-
are important because they can be related to the details tron affinity. When the negative ion thus formed is stable,
of electronic configuration. For example, the experimen- energy is released and the electron affinity has a nega-
tal values for ionization energy give evidence of energy tive sign. When the ion is unstable, the electron affinity is
differences between s and p orbitals in the same electron positive. A comparison of Figs. 5 and 6 reveals that the
shell and the stability associated with half-filled and fully variation of ionization potentials is much greater than that
filled subshells. In fact, some of the best evidence for the of the related electron affinities, and this is why ionization
correctness of the quantum mechanical model of the atom potentials serve better as singular indices of the electronic
comes from such data. natures of different elements.
C. Electron Affinity
V. THE NEW VALENCE BOND
A good deal of energy is required to form positive ions INTERPRETATION OF CHEMISTRY
from the elements of Group 17. On the other hand, fluo-
rine and the other halogens tend to gain one electron per Vultures are flying over chemistry. In Horgan’s The End of
atom in chemical reactions—a process called reduction. Science, chemistry assumes a secondary role smashed by
The negative ions that result are, once more, isoelectronic the two behemoths of biology and physics as they them-
with the adjacent rare gas atoms. Thus, a chlorine atom, selves approach extinction. In Ideas in Chemistry, Knight
2
6
2
2
5
with a 1s 2 s 2 p 3s 3p electron configuration, becomes proclaims the dicipline to be far past its golden days.
−
converted to a chloride ion, Cl , with the stable configura- Chemistry’s icon Linus Pauling, in 1992, concurs: “I felt