Page 82 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 82
P1: FQP/LPB P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN003D-147 June 13, 2001 22:58
Coordination Compounds 753
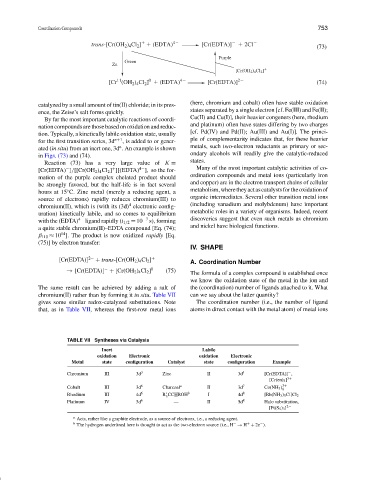
trans-[Cr(OH 2 ) 4 Cl 2 ] (EDTA) 4 [Cr(EDTA)] 2Cl
(73)
Purple
Green
Zn
[Cr(OH 2 ) 4 Cl 2 ]
0
11
[Cr (OH 2 ) 4 Cl 2 ] (EDTA) 4 [Cr(EDTA)] 2 (74)
catalyzed by a small amount of tin(II) chloride; in its pres- (here, chromium and cobalt) often have stable oxidation
ence, the Zeise’s salt forms quickly. states separated by a single electron [cf. Fe(III) and Fe(II);
By far the most important catalytic reactions of coordi- Cu(II) and Cu(I)], their heavier congeners (here, rhodium
nation compounds are those based on oxidation and reduc- and platinum) often have states differing by two charges
[cf. Pd(IV) and Pd(II); Au(III) and Au(I)]. The princi-
tion. Typically, a kinetically labile oxidation state, usually
for the first transition series, 3d n +1 , is added to or gener- ple of complementarity indicates that, for these heavier
n
ated (in situ) from an inert one, 3d . An example is shown metals, such two-electron reductants as primary or sec-
ondary alcohols will readily give the catalytic-reduced
in Figs. (73) and (74).
states.
Reaction (73) has a very large value of K =
Many of the most important catalytic activities of co-
+
[Cr(EDTA) ]/[[Cr(OH 2 ) 4 Cl 2 ] ][(EDTA) ], so the for-
4−
−
ordination compounds and metal ions (particularly iron
mation of the purple complex chelated product should
and copper) are in the electron transport chains of cellular
be strongly favored, but the half-life is in fact several
metabolism,wheretheyactascatalystsfortheoxidationof
◦
hours at 15 C. Zinc metal (merely a reducing agent, a
organic intermediates. Several other transition metal ions
source of electrons) rapidly reduces chromium(III) to
4
chromium(II), which is (with its (3d) electronic config- (including vanadium and molybdenum) have important
metabolic roles in a variety of organisms. Indeed, recent
uration) kinetically labile, and so comes to equilibrium
with the (EDTA) 4− ligand rapidly (t 1/2 = 10 −7 s), forming discoveries suggest that even such metals as chromium
and nickel have biological functions.
a quite stable chromium(II)–EDTA compound [Eq. (74);
14
β 110 ≈ 10 ]. The product is now oxidized rapidly [Eq.
(75)] by electron transfer:
IV. SHAPE
[Cr(EDTA)] 2− + trans-[Cr(OH 2 ) 4 Cl 2 ] + A. Coordination Number
→ [Cr(EDTA)] + [Cr(OH 2 ) 4 Cl 2 ] 0 (75)
−
The formula of a complex compound is established once
we know the oxidation state of the metal in the ion and
The same result can be achieved by adding a salt of the (coordination) number of ligands attached to it. What
chromium(II) rather than by forming it in situ. Table VII can we say about the latter quantity?
gives some similar redox-catalyzed substitutions. Note The coordination number (i.e., the number of ligand
that, as in Table VII, whereas the first-row metal ions atoms in direct contact with the metal atom) of metal ions
TABLE VII Syntheses via Catalysis
Inert Labile
oxidation Electronic oxidation Electronic
Metal state configuration Catalyst state configuration Example
−
Chromium III 3d 3 Zinc II 3d 4 [Cr(EDTA)] ,
[Cr(en) 3 ] 3+
Cobalt III 3d 6 Charcoal a II 3d 7 Co(NH 3 ) 3+
6
Rhodium III 4d 6 R CCHROH b I 4d 8 [Rh(NH 3 ) 5 Cl]Cl 2
3
Platinum IV 5d 6 — II 5d 8 Halo substitution,
[Pt(S 5 ) 3 ] 2−
a Acts, rather like a graphite electrode, as a source of electrons, i.e., a reducing agent.
b The hydrogen underlined here is thought to act as the two-electron source (i.e., H → H + 2e ).
−
−
+