Page 86 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 86
P1: FQP/LPB P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN003D-147 June 13, 2001 22:58
Coordination Compounds 757
(45) is the same as that in the reactant (43). This chem-
ical transformation proved that the two chiralities (hand-
edness of propeller) are related and is called a chemi-
cal correlation of configuration. Other examples include
Eqs. (78)–(80):
(+)-cis-[Co(en) 2 (NCS) 2 ] +
→ (+)-cis-[Co(en) 2 (NH 3 ) 2 ] 3+ (78)
(79)
(80)
This is a neat dissymmetric synthesis of the product,
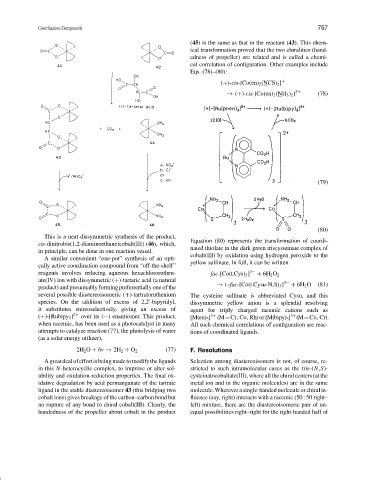
Equation (80) represents the transformation of coordi-
cis-dinitrobis(1,2-diaminoethane)cobalt(III) (46), which,
nated thiolate in the dark green triscysteinate complex of
in principle, can be done in one reaction vessel.
cobalt(III) by oxidation using hydrogen peroxide to the
A similar convenient “one-pot” synthesis of an opti-
yellow sulfinate. In full, it can be written
cally active coordination compound from “off-the-shelf”
reagents involves reducing aqueous hexachlororuthen- fac-[Co(LCys) 3 ] 3− + 6H 2 O 2
ate(IV) ion with dissymmetric (+)-tartaric acid (a natural
→ L-fac-[Co(LCysu-N,S) 3 ] 3− + 6H 2 O (81)
product) and presumably forming preferentially one of the
several possible diastereoisomeric (+)-tartratoruthenium The cysteine sulfinate is abbreviated Cysu, and this
species. On the addition of excess of 2,2 -bipyridyl, dissymmetric yellow anion is a splendid resolving
it substitutes stereoselectively, giving an excess of agent for triply charged racemic cations such as
(+)-[Rubipy 3 ] 2+ over its (−) enantiomer. This product, [M(en) 3 ] 3+ (M=Cr, Co, Rh) or [M(bipy) 3 ] (M=Co, Cr).
3+
when racemic, has been used as a photocatalyst in many All such chemical correlations of configuration are reac-
attempts to catalyze reaction (77), the photolysis of water tions of coordinated ligands.
(as a solar energy utilizer),
(77)
2H 2 O + hv → 2H 2 + O 2 F. Resolutions
Agreatdealofeffortisbeingmadetomodifytheligands Selection among diastereoisomers is not, of course, re-
in this N-heterocyclic complex, to improve or alter sol- stricted to such intramolecular cases as the tris-(N,S)-
ubility and oxidation-reduction properties. The final ox- cysteinatocobaltate(III), where all the chiral centers (at the
idative degradation by acid permanganate of the tartrate metal ion and in the organic molecules) are in the same
ligand in the stable diastereoisomer 43 (this bridging two molecule.Whereverasingle-handedmoleculeorchiralin-
cobalt ions) gives breakage of the carbon–carbon bond but fluence (say, right) interacts with a racemic (50 : 50 right–
no rupture of any bond to chiral cobalt(III). Clearly, the left) mixture, there are the diastereoisomeric pair of un-
handedness of the propeller about cobalt in the product equal possibilities right–right for the right-handed half of