Page 7 - Academic Press Encyclopedia of Physical Science and Technology 3rd Molecular Biology
P. 7
P1: GQQ Revised Pages
Encyclopedia of Physical Science and Technology EN002G-90 May 17, 2001 20:42
Cell Death (Apoptosis) 545
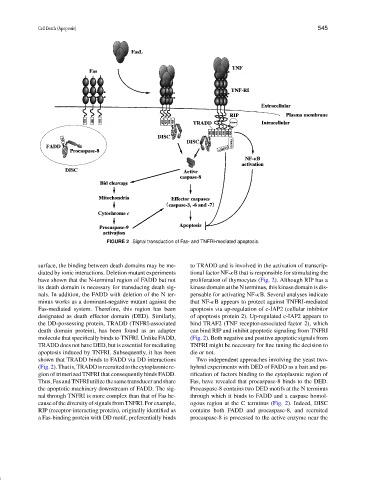
FIGURE 2 Signal transduction of Fas- and TNFRI-mediated apoptosis.
surface, the binding between death domains may be me- to TRADD and is involved in the activation of transcrip-
diated by ionic interactions. Deletion mutant experiments tional factor NF-κB that is responsible for stimulating the
have shown that the N-terminal region of FADD but not proliferation of thymocytes (Fig. 2). Although RIP has a
its death domain is necessary for transducing death sig- kinase domain at the N terminus, this kinase domain is dis-
nals. In addition, the FADD with deletion of the N ter- pensable for activating NF-κB. Several analyses indicate
minus works as a dominant-negative mutant against the that NF-κB appears to protect against TNFRI-mediated
Fas-mediated system. Therefore, this region has been apoptosis via up-regulation of c-IAP2 (cellular inhibitor
designated as death effector domain (DED). Similarly, of apoptosis protein 2). Up-regulated c-IAP2 appears to
the DD-possessing protein, TRADD (TNFRI-associated bind TRAF2 (TNF receptor-associated factor 2), which
death domain protein), has been found as an adapter can bind RIP and inhibit apoptotic signaling from TNFRI
molecule that specifically binds to TNFRI. Unlike FADD, (Fig. 2). Both negative and positive apoptotic signals from
TRADD does not have DED, but is essential for mediating TNFRI might be necessary for fine tuning the decision to
apoptosis induced by TNFRI. Subsequently, it has been die or not.
shown that TRADD binds to FADD via DD interactions Two independent approaches involving the yeast two-
(Fig.2).Thatis,TRADDisrecruitedtothecytoplasmicre- hybrid experiments with DED of FADD as a bait and pu-
gion of trimerized TNFRI that consequently binds FADD. rification of factors binding to the cytoplasmic region of
Thus,FasandTNFRIutilize thesametransducerand share Fas, have revealed that procaspase-8 binds to the DED.
the apoptotic machinery downstream of FADD. The sig- Procaspase-8 contains two DED motifs at the N terminus
nal through TNFRI is more complex than that of Fas be- through which it binds to FADD and a caspase homol-
cause of the diversity of signals from TNFRI. For example, ogous region at the C terminus (Fig. 2). Indeed, DISC
RIP (receptor-interacting protein), originally identified as contains both FADD and procaspase-8, and recruited
a Fas-binding protein with DD motif, preferentially binds procaspase-8 is processed to the active enzyme near the