Page 299 - Engineering Plastics Handbook
P. 299
Polyamide-imide (PAI) 259
Polyamide-imide Polymerization Chemistry
There are currently two popular commercial methods to synthesize
polyamide-imides. One is the acid chloride route, and the other is the
isocyanate route. The synthesis method used will determine and limit
to some extent the applications in which the resultant polymer is used.
The chemistry of these two methods is outlined below.
Acid chloride route
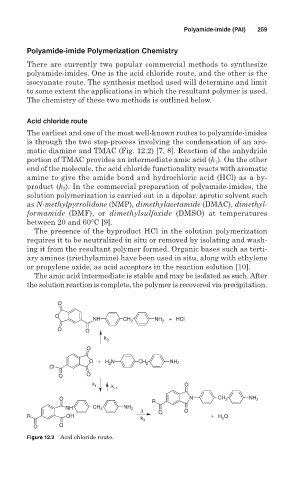
The earliest and one of the most well-known routes to polyamide-imides
is through the two step-process involving the condensation of an aro-
matic diamine and TMAC (Fig. 12.2) [7, 8]. Reaction of the anhydride
portion of TMAC provides an intermediate amic acid (k ). On the other
1
end of the molecule, the acid chloride functionality reacts with aromatic
amine to give the amide bond and hydrochloric acid (HCl) as a by-
product (k ). In the commercial preparation of polyamide-imides, the
2
solution polymerization is carried out in a dipolar, aprotic solvent such
as N-methylpyrrolidone (NMP), dimethylacetamide (DMAC), dimethyl-
formamide (DMF), or dimethylsulfoxide (DMSO) at temperatures
between 20 and 60°C [9].
The presence of the byproduct HCl in the solution polymerization
requires it to be neutralized in situ or removed by isolating and wash-
ing it from the resultant polymer formed. Organic bases such as terti-
ary amines (triethylamine) have been used in situ, along with ethylene
or propylene oxide, as acid acceptors in the reaction solution [10].
The amic acid intermediate is stable and may be isolated as such. After
the solution reaction is complete, the polymer is recovered via precipitation.
O
O
NH CH 2 NH 2 + HCl
O O
k
2
O
O + H N CH 2 NH 2
2
Cl
O O
k −1
k 1 O
O N CH NH 2
R 2
NH CH 2 NH 2 ∆ O
R OH O + H O
k 3 2
O O
Figure 12.2 Acid chloride route.