Page 301 - Engineering Plastics Handbook
P. 301
Polyamide-imide (PAI) 261
O
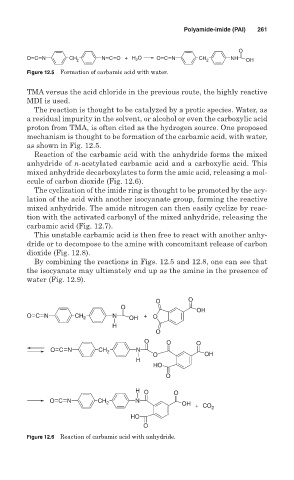
O C N CH N C O + H O O C N CH NH
2 2 2 OH
Figure 12.5 Formation of carbamic acid with water.
TMA versus the acid chloride in the previous route, the highly reactive
MDI is used.
The reaction is thought to be catalyzed by a protic species. Water, as
a residual impurity in the solvent, or alcohol or even the carboxylic acid
proton from TMA, is often cited as the hydrogen source. One proposed
mechanism is thought to be formation of the carbamic acid, with water,
as shown in Fig. 12.5.
Reaction of the carbamic acid with the anhydride forms the mixed
anhydride of n-acetylated carbamic acid and a carboxylic acid. This
mixed anhydride decarboxylates to form the amic acid, releasing a mol-
ecule of carbon dioxide (Fig. 12.6).
The cyclization of the imide ring is thought to be promoted by the acy-
lation of the acid with another isocyanate group, forming the reactive
mixed anhydride. The amide nitrogen can then easily cyclize by reac-
tion with the activated carbonyl of the mixed anhydride, releasing the
carbamic acid (Fig. 12.7).
This unstable carbamic acid is then free to react with another anhy-
dride or to decompose to the amine with concomitant release of carbon
dioxide (Fig. 12.8).
By combining the reactions in Figs. 12.5 and 12.8, one can see that
the isocyanate may ultimately end up as the amine in the presence of
water (Fig. 12.9).
O O
O
OH
O C N CH 2 N OH + O
H
O
O O O
O C N CH 2 N
O OH
H
HO
O
H O O
O C N CH 2 N OH + CO 2
HO
O
Figure 12.6 Reaction of carbamic acid with anhydride.