Page 218 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 218
Fluid-rock interactions 201
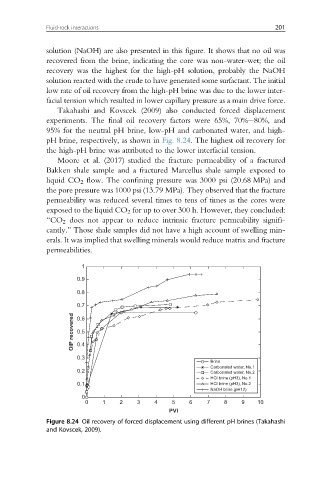
solution (NaOH) are also presented in this figure. It shows that no oil was
recovered from the brine, indicating the core was non-water-wet; the oil
recovery was the highest for the high-pH solution, probably the NaOH
solution reacted with the crude to have generated some surfactant. The initial
low rate of oil recovery from the high-pH brine was due to the lower inter-
facial tension which resulted in lower capillary pressure as a main drive force.
Takahashi and Kovscek (2009) also conducted forced displacement
experiments. The final oil recovery factors were 65%, 70%e80%, and
95% for the neutral pH brine, low-pH and carbonated water, and high-
pH brine, respectively, as shown in Fig. 8.24. The highest oil recovery for
the high-pH brine was attributed to the lower interfacial tension.
Moore et al. (2017) studied the fracture permeability of a fractured
Bakken shale sample and a fractured Marcellus shale sample exposed to
liquid CO 2 flow. The confining pressure was 3000 psi (20.68 MPa) and
the pore pressure was 1000 psi (13.79 MPa). They observed that the fracture
permeability was reduced several times to tens of times as the cores were
exposed to the liquid CO 2 for up to over 300 h. However, they concluded:
“CO 2 does not appear to reduce intrinsic fracture permeability signifi-
cantly.” Those shale samples did not have a high account of swelling min-
erals. It was implied that swelling minerals would reduce matrix and fracture
permeabilities.
1
0.9
0.8
0.7
OlP recovered 0.6
0.5
0.4
0.3
Brine
Carbonated water, No.1
0.2 Carbonated water, No.2
HCI brine (pH3), No.1
0.1 HCI brine (pH3), No.2
NaOH brine (pH12)
0
0 1 2 3 4 5 6 7 8 9 10
PVI
Figure 8.24 Oil recovery of forced displacement using different pH brines (Takahashi
and Kovscek, 2009).