Page 220 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 220
Fluid-rock interactions 203
showed that water at different alkaline solutions had different degree of reac-
tions with shale rocks. The spontaneous oil recovery from cores (a), (b), and
(c) were 17%, 15%, and 20%, respectively (Morsy and Sheng, 2013c). The
core (c) had the highest oil recovery with it being most damaged or reacted.
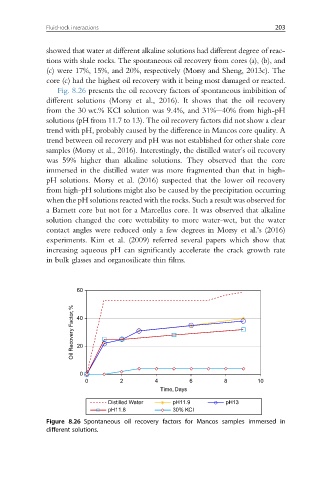
Fig. 8.26 presents the oil recovery factors of spontaneous imbibition of
different solutions (Morsy et al., 2016). It shows that the oil recovery
from the 30 wt.% KCl solution was 9.4%, and 31%e40% from high-pH
solutions (pH from 11.7 to 13). The oil recovery factors did not show a clear
trend with pH, probably caused by the difference in Mancos core quality. A
trend between oil recovery and pH was not established for other shale core
samples (Morsy et al., 2016). Interestingly, the distilled water’s oil recovery
was 59% higher than alkaline solutions. They observed that the core
immersed in the distilled water was more fragmented than that in high-
pH solutions. Morsy et al. (2016) suspected that the lower oil recovery
from high-pH solutions might also be caused by the precipitation occurring
when the pH solutions reacted with the rocks. Such a result was observed for
a Barnett core but not for a Marcellus core. It was observed that alkaline
solution changed the core wettability to more water-wet, but the water
contact angles were reduced only a few degrees in Morsy et al.‘s (2016)
experiments. Kim et al. (2009) referred several papers which show that
increasing aqueous pH can significantly accelerate the crack growth rate
in bulk glasses and organosilicate thin films.
60
Oil Recovery Factor, % 40
20
0
0 2 4 6 8 10
Time, Days
Distilled Water pH11.9 pH13
pH11.8 30% KCI
Figure 8.26 Spontaneous oil recovery factors for Mancos samples immersed in
different solutions.