Page 225 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 225
208 Enhanced Oil Recovery in Shale and Tight Reservoirs
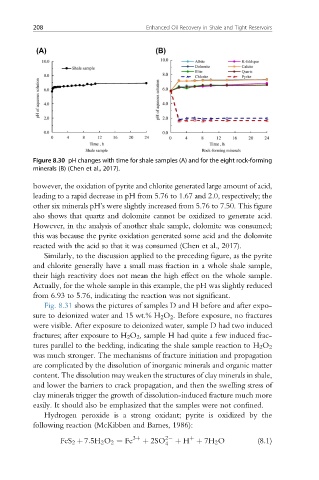
Figure 8.30 pH changes with time for shale samples (A) and for the eight rock-forming
minerals (B) (Chen et al., 2017).
however, the oxidation of pyrite and chlorite generated large amount of acid,
leading to a rapid decrease in pH from 5.76 to 1.67 and 2.0, respectively; the
other six minerals pH’s were slightly increased from 5.76 to 7.50. This figure
also shows that quartz and dolomite cannot be oxidized to generate acid.
However, in the analysis of another shale sample, dolomite was consumed;
this was because the pyrite oxidation generated some acid and the dolomite
reacted with the acid so that it was consumed (Chen et al., 2017).
Similarly, to the discussion applied to the preceding figure, as the pyrite
and chlorite generally have a small mass fraction in a whole shale sample,
their high reactivity does not mean the high effect on the whole sample.
Actually, for the whole sample in this example, the pH was slightly reduced
from 6.93 to 5.76, indicating the reaction was not significant.
Fig. 8.31 shows the pictures of samples D and H before and after expo-
sure to deionized water and 15 wt.% H 2 O 2 . Before exposure, no fractures
were visible. After exposure to deionized water, sample D had two induced
fractures; after exposure to H 2 O 2 , sample H had quite a few induced frac-
tures parallel to the bedding, indicating the shale sample reaction to H 2 O 2
was much stronger. The mechanisms of fracture initiation and propagation
are complicated by the dissolution of inorganic minerals and organic matter
content. The dissolution may weaken the structures of clay minerals in shale,
and lower the barriers to crack propagation, and then the swelling stress of
clay minerals trigger the growth of dissolution-induced fracture much more
easily. It should also be emphasized that the samples were not confined.
Hydrogen peroxide is a strong oxidant; pyrite is oxidized by the
following reaction (McKibben and Barnes, 1986):
þ
FeS 2 þ 7:5H 2 O 2 ¼ Fe 3þ þ 2SO 2 þ H þ 7H 2 O (8.1)
4