Page 226 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 226
Fluid-rock interactions 209
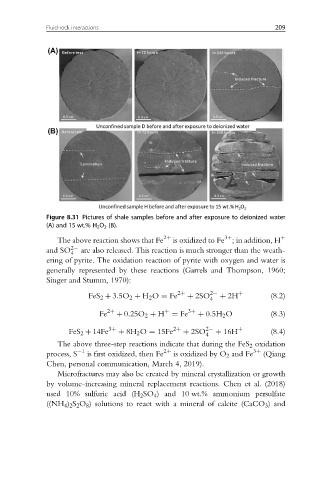
Figure 8.31 Pictures of shale samples before and after exposure to deionized water
(A) and 15 wt.% H 2 O 2 (B).
3þ
The above reaction shows that Fe 2þ is oxidized to Fe ; in addition, H þ
and SO 2 are also released. This reaction is much stronger than the weath-
4
ering of pyrite. The oxidation reaction of pyrite with oxygen and water is
generally represented by these reactions (Garrels and Thompson, 1960;
Singer and Stumm, 1970):
FeS 2 þ 3:5O 2 þ H 2 O ¼ Fe 2þ þ 2SO 2 þ 2H þ (8.2)
4
þ
Fe 2þ þ 0:25O 2 þ H ¼ Fe 3þ þ 0:5H 2 O (8.3)
FeS 2 þ 14Fe 3þ þ 8H 2 O ¼ 15Fe 2þ þ 2SO 2 þ 16H þ (8.4)
4
The above three-step reactions indicate that during the FeS 2 oxidation
1 2þ 3þ
process, S is first oxidized, then Fe is oxidized by O 2 and Fe (Qiang
Chen, personal communication, March 4, 2019).
Microfractures may also be created by mineral crystallization or growth
by volume-increasing mineral replacement reactions. Chen et al. (2018)
used 10% sulfuric acid (H 2 SO 4 ) and 10 wt.% ammonium persulfate
((NH 4 ) 2 S 2 O 8 ) solutions to react with a mineral of calcite (CaCO 3 ) and