Page 243 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 243
226 Enhanced Oil Recovery in Shale and Tight Reservoirs
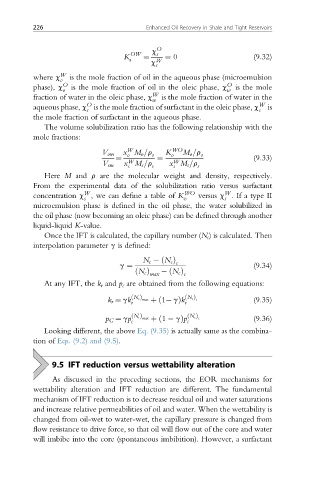
c O
OW s
K s ¼ ¼ 0 (9.32)
c W
s
where c W is the mole fraction of oil in the aqueous phase (microemulsion
o
phase), c O is the mole fraction of oil in the oleic phase, c O is the mole
o w
fraction of water in the oleic phase, c W is the mole fraction of water in the
w
O
aqueous phase, c is the mole fraction of surfactant in the oleic phase, c W is
s
s
the mole fraction of surfactant in the aqueous phase.
The volume solubilization ratio has the following relationship with the
mole fractions:
W
V om x M o =r o K o WO M o =r o
o
¼ ¼ (9.33)
W
W
V sm x M s =r s x M s =r s
s
s
Here M and r are the molecular weight and density, respectively.
From the experimental data of the solubilization ratio versus surfactant
W
W
concentration c ,we can define a table of K WO versus c . If a type II
s o s
microemulsion phase is defined in the oil phase, the water solubilized in
the oil phase (now becoming an oleic phase) can be defined through another
liquid-liquid K-value.
Once the IFT is calculated, the capillary number (N c ) is calculated. Then
interpolation parameter g is defined:
N c ðN c Þ c
g ¼ (9.34)
ðN c Þ ðN c Þ
max c
At any IFT, the k r and p c are obtained from the following equations:
k r ¼ gk ðN c Þ max þð1 gÞk ðN c Þ c (9.35)
r r
p C ¼ gp ðN c Þ max þð1 gÞp ðN c Þ c (9.36)
c c
Looking different, the above Eq. (9.35) is actually same as the combina-
tion of Eqs. (9.2) and (9.5).
9.5 IFT reduction versus wettability alteration
As discussed in the preceding sections, the EOR mechanisms for
wettability alteration and IFT reduction are different. The fundamental
mechanism of IFT reduction is to decrease residual oil and water saturations
and increase relative permeabilities of oil and water. When the wettability is
changed from oil-wet to water-wet, the capillary pressure is changed from
flow resistance to drive force, so that oil will flow out of the core and water
will imbibe into the core (spontaneous imbibition). However, a surfactant