Page 257 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 257
EOR mechanisms of wettability alteration and its comparison with IFT 239
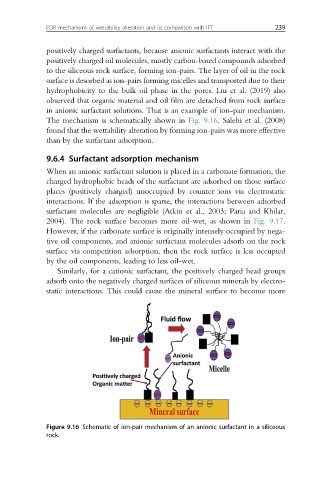
positively charged surfactants, because anionic surfactants interact with the
positively charged oil molecules, mostly carbon-based compounds adsorbed
to the siliceous rock surface, forming ion-pairs. The layer of oil in the rock
surface is desorbed as ion-pairs forming micelles and transported due to their
hydrophobicity to the bulk oil phase in the pores. Liu et al. (2019) also
observed that organic material and oil film are detached from rock surface
in anionic surfactant solutions. That is an example of ion-pair mechanism.
The mechanism is schematically shown in Fig. 9.16. Salehi et al. (2008)
found that the wettability alteration by forming ion-pairs was more effective
than by the surfactant adsorption.
9.6.4 Surfactant adsorption mechanism
When an anionic surfactant solution is placed in a carbonate formation, the
charged hydrophobic heads of the surfactant are adsorbed on those surface
places (positively charged) unoccupied by counter ions via electrostatic
interactions. If the adsorption is sparse, the interactions between adsorbed
surfactant molecules are negligible (Atkin et al., 2003; Paria and Khilar,
2004). The rock surface becomes more oil-wet, as shown in Fig. 9.17.
However, if the carbonate surface is originally intensely occupied by nega-
tive oil components, and anionic surfactant molecules adsorb on the rock
surface via competition adsorption, then the rock surface is less occupied
by the oil components, leading to less oil-wet.
Similarly, for a cationic surfactant, the positively charged head groups
adsorb onto the negatively charged surfaces of siliceous minerals by electro-
static interactions. This could cause the mineral surface to become more
Figure 9.16 Schematic of ion-pair mechanism of an anionic surfactant in a siliceous
rock.