Page 255 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 255
EOR mechanisms of wettability alteration and its comparison with IFT 237
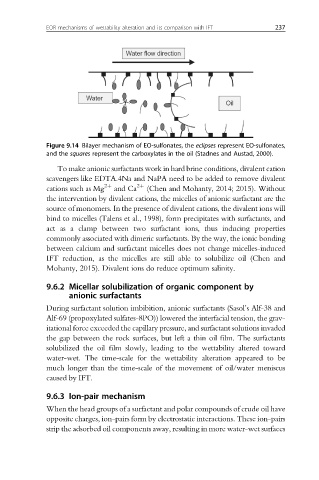
Figure 9.14 Bilayer mechanism of EO-sulfonates, the eclipses represent EO-sulfonates,
and the squares represent the carboxylates in the oil (Stadnes and Austad, 2000).
To make anionic surfactants work in hard brine conditions, divalent cation
scavengers like EDTA.4Na and NaPA need to be added to remove divalent
cations such as Mg 2þ and Ca 2þ (Chen and Mohanty, 2014; 2015). Without
the intervention by divalent cations, the micelles of anionic surfactant are the
source of monomers. In the presence of divalent cations, the divalent ions will
bind to micelles (Talens et al., 1998), form precipitates with surfactants, and
act as a clamp between two surfactant ions, thus inducing properties
commonly associated with dimeric surfactants. By the way, the ionic bonding
between calcium and surfactant micelles does not change micelles-induced
IFT reduction, as the micelles are still able to solubilize oil (Chen and
Mohanty, 2015). Divalent ions do reduce optimum salinity.
9.6.2 Micellar solubilization of organic component by
anionic surfactants
During surfactant solution imbibition, anionic surfactants (Sasol’sAlf-38 and
Alf-69 (propoxylated sulfates-8PO)) lowered the interfacial tension, the grav-
itational force exceeded the capillary pressure, and surfactant solutions invaded
the gap between the rock surfaces, but left a thin oil film. The surfactants
solubilized the oil film slowly, leading to the wettability altered toward
water-wet. The time-scale for the wettability alteration appeared to be
much longer than the time-scale of the movement of oil/water meniscus
caused by IFT.
9.6.3 Ion-pair mechanism
When the head groups of a surfactant and polar compounds of crude oil have
opposite charges, ion-pairs form by electrostatic interactions. These ion-pairs
strip the adsorbed oil components away, resulting in more water-wet surfaces