Page 259 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 259
EOR mechanisms of wettability alteration and its comparison with IFT 241
Figure 9.19 Schematic of monolayer mechanism of wettability alteration.
in Fig. 9.19A. This process is reversible because of the week hydrophobic
interactions (Salehi et al., 2008; Standnes et al., 2002). When the surfactant
is added, surfactant molecules tend to diffuse to the interfaces between
isolated oil droplets and water due to the surface tension gradient or the
Gibbs-Marangoni effect (Sheng, 2013d). The surfactant molecules displace
the oil attached to the rock surface, and the isolated oil droplets tend to
roll up slowly and eventually detach from the surface, as shown in
Fig. 9.19B.
9.6.6 Effect of IFT reduction on wettability alteration
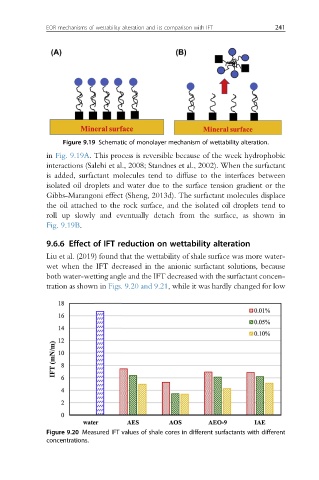
Liu et al. (2019) found that the wettability of shale surface was more water-
wet when the IFT decreased in the anionic surfactant solutions, because
both water-wetting angle and the IFT decreased with the surfactant concen-
tration as shown in Figs. 9.20 and 9.21, while it was hardly changed for low
Figure 9.20 Measured IFT values of shale cores in different surfactants with different
concentrations.