Page 284 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 284
EOR mechanisms of wettability alteration and its comparison with IFT 261
(F) (G)
3000 2000
0 h 0 h
12 h 12 h
24 h 24 h
48 h 48 h
96 h 1500 96 h
2000 192 h 192 h
240 h
240 h
Amplitude AOS-0.01% Amplitude 1000
1000 AOS-0.1%
500
0 0
0.01 0.1 1 10 100 1000 0.01 0.1 1 10 100 1000
T relaxation time/ms T relaxation time/ms
(H) (I)
2500 3000
0 h 0 h
12 h 12 h
24 h 24 h
2000 48 h 48 h
96 h 96 h
192 h 2000 192 h
240 h
240 h
Amplitude Amplitude
1500
1000
AES-0.01% 1000 AES-0.1%
500
0 0
0.01 0.1 1 10 100 1000 0.01 0.1 1 10 100 1000
T relaxation time/ms T relaxation time/ms
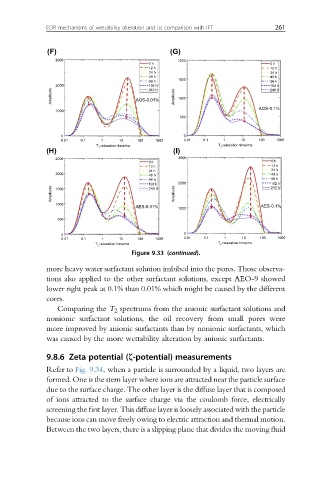
Figure 9.33 (continued).
more heavy water surfactant solution imbibed into the pores. Those observa-
tions also applied to the other surfactant solutions, except AEO-9 showed
lower right peak at 0.1% than 0.01% which might be caused by the different
cores.
Comparing the T 2 spectrums from the anionic surfactant solutions and
nonionic surfactant solutions, the oil recovery from small pores were
more improved by anionic surfactants than by nonionic surfactants, which
was caused by the more wettability alteration by anionic surfactants.
9.8.6 Zeta potential (z-potential) measurements
Refer to Fig. 9.34, when a particle is surrounded by a liquid, two layers are
formed. One is the stern layer where ions are attracted near the particle surface
due to the surface charge. The other layer is the diffuse layer that is composed
of ions attracted to the surface charge via the coulomb force, electrically
screening the first layer. This diffuse layer is loosely associated with the particle
because ions can move freely owing to electric attraction and thermal motion.
Between the two layers, there is a slipping plane that divides the moving fluid