Page 482 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 482
446 Enhanced Oil Recovery in Shale and Tight Reservoirs
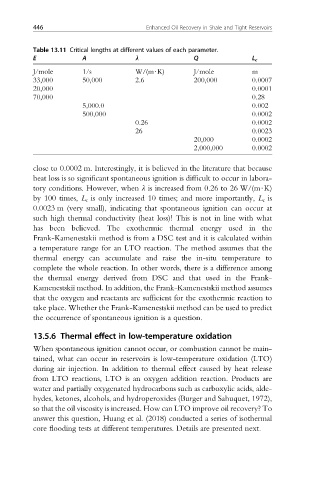
Table 13.11 Critical lengths at different values of each parameter.
E A l Q L c
J/mole 1/s W/(m$K) J/mole m
33,000 50,000 2.6 200,000 0.0007
20,000 0.0001
70,000 0.28
5,000.0 0.002
500,000 0.0002
0.26 0.0002
26 0.0023
20,000 0.0002
2,000,000 0.0002
close to 0.0002 m. Interestingly, it is believed in the literature that because
heat loss is so significant spontaneous ignition is difficult to occur in labora-
tory conditions. However, when l is increased from 0.26 to 26 W/(m$K)
by 100 times, L c is only increased 10 times; and more importantly, L c is
0.0023 m (very small), indicating that spontaneous ignition can occur at
such high thermal conductivity (heat loss)! This is not in line with what
has been believed. The exothermic thermal energy used in the
Frank-Kamenestskii method is from a DSC test and it is calculated within
a temperature range for an LTO reaction. The method assumes that the
thermal energy can accumulate and raise the in-situ temperature to
complete the whole reaction. In other words, there is a difference among
the thermal energy derived from DSC and that used in the Frank-
Kamenestskii method. In addition, the Frank-Kamenestskii method assumes
that the oxygen and reactants are sufficient for the exothermic reaction to
take place. Whether the Frank-Kamenestskii method can be used to predict
the occurrence of spontaneous ignition is a question.
13.5.6 Thermal effect in low-temperature oxidation
When spontaneous ignition cannot occur, or combustion cannot be main-
tained, what can occur in reservoirs is low-temperature oxidation (LTO)
during air injection. In addition to thermal effect caused by heat release
from LTO reactions, LTO is an oxygen addition reaction. Products are
water and partially oxygenated hydrocarbons such as carboxylic acids, alde-
hydes, ketones, alcohols, and hydroperoxides (Burger and Sahuquet, 1972),
so that the oil viscosity is increased. How can LTO improve oil recovery? To
answer this question, Huang et al. (2018) conducted a series of isothermal
core flooding tests at different temperatures. Details are presented next.