Page 155 - Entrophy Analysis in Thermal Engineering Systems
P. 155
150 Entropy Analysis in Thermal Engineering Systems
where G denotes the Gibbs function defined as G¼H TS, H is the
enthalpy of the system, T the temperature, and S the entropy.
A second class of models widely used for prediction of the composition
of a chemical reaction employs the conservation of energy, conservation of
elements (e.g., carbon, hydrogen, oxygen), and the Gibbs criterion, but they
disregard the physiochemical processes. The main task of this chapter is to
evaluate the validity of Eq. (10.1).
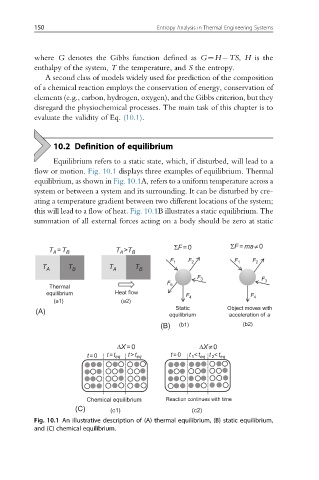
10.2 Definition of equilibrium
Equilibrium refers to a static state, which, if disturbed, will lead to a
flow or motion. Fig. 10.1 displays three examples of equilibrium. Thermal
equilibrium, as shown in Fig. 10.1A, refers to a uniform temperature across a
system or between a system and its surrounding. It can be disturbed by cre-
ating a temperature gradient between two different locations of the system;
this will lead to a flow of heat. Fig. 10.1B illustrates a static equilibrium. The
summation of all external forces acting on a body should be zero at static
T =T B T >T B ΣF=0 ΣF =ma≠ 0
A
A
F 1 F 2 F 1 F 2
T A T B T A T B
F 3
F 3
F 5
Thermal
equilibrium Heat flow
F 4 F 4
(a1) (a2)
Static Object moves with
(A)
equilibrium acceleration of a
(B) (b1) (b2)
ΔX=0 ΔX ≠ 0
t =0 t =t eq t >t eq t =0 t 1 <t eq t <t eq
2
Chemical equilibrium Reaction continues with time
(C) (c1) (c2)
Fig. 10.1 An illustrative description of (A) thermal equilibrium, (B) static equilibrium,
and (C) chemical equilibrium.