Page 159 - Entrophy Analysis in Thermal Engineering Systems
P. 159
154 Entropy Analysis in Thermal Engineering Systems
N 2
CO 2
CO
CH 4
H 2
−100 −80 −60 −40 −20 0 20 40 60 80 100
Average % error
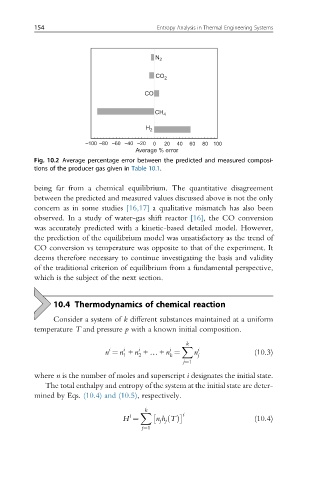
Fig. 10.2 Average percentage error between the predicted and measured composi-
tions of the producer gas given in Table 10.1.
being far from a chemical equilibrium. The quantitative disagreement
between the predicted and measured values discussed above is not the only
concern as in some studies [16,17] a qualitative mismatch has also been
observed. In a study of water-gas shift reactor [16], the CO conversion
was accurately predicted with a kinetic-based detailed model. However,
the prediction of the equilibrium model was unsatisfactory as the trend of
CO conversion vs temperature was opposite to that of the experiment. It
deems therefore necessary to continue investigating the basis and validity
of the traditional criterion of equilibrium from a fundamental perspective,
which is the subject of the next section.
10.4 Thermodynamics of chemical reaction
Consider a system of k different substances maintained at a uniform
temperature T and pressure p with a known initial composition.
k
X
i
i
i
i
n ¼ n + n + … + n ¼ i (10.3)
1 2 k n j
j¼1
where n is the number of moles and superscript i designates the initial state.
The total enthalpy and entropy of the system at the initial state are deter-
mined by Eqs. (10.4) and (10.5), respectively.
k
X
i
H ¼ n j h j TðÞ (10.4)
i
j¼1