Page 164 - Entrophy Analysis in Thermal Engineering Systems
P. 164
Entropy and chemical equilibrium 159
k k
X X
f
f
ð
G ξðÞ ¼ G ξ ðÞ ¼ n + a j ξ h j TðÞ T s s j T, pÞ (10.19)
f
i
m m, j j
j¼1 j¼1
For a reactive system with a fixed initial state, Eq. (10.19) states that G m is
f
only a function of ξ. Also, since G m is constant, the entropy generation Φ is
i
dependent on G m —this can be deduced from Eq. (10.10). We therefore
f
conclude that Φ is also a function of ξ only.
10.5.1 Methane steam reforming
Let us now consider a simple example where the reactants are methane and
steam and the products are hydrogen and carbon monoxide.
CH 4 +H 2 O ! 3H 2 + CO (10.20)
The reaction takes place at an elevated temperature T and 1bar. The sto-
¼ 1, a H 2 O ¼ 1,
chiometric coefficients of reaction (10.20) are: a CH 4
¼3, and a CO ¼1. From Eq. (10.18), we then find a¼2. Fig. 10.3 depicts
a H 2
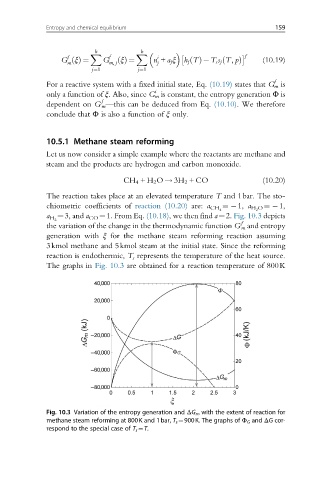
the variation of the change in the thermodynamic function G m and entropy
f
generation with ξ for the methane steam reforming reaction assuming
3kmol methane and 5kmol steam at the initial state. Since the reforming
reaction is endothermic, T s represents the temperature of the heat source.
The graphs in Fig. 10.3 are obtained for a reaction temperature of 800K
Fig. 10.3 Variation of the entropy generation and ΔG m with the extent of reaction for
methane steam reforming at 800K and 1bar, T s ¼900K. The graphs of Φ G and ΔG cor-
respond to the special case of T s ¼T.