Page 169 - Entrophy Analysis in Thermal Engineering Systems
P. 169
164 Entropy Analysis in Thermal Engineering Systems
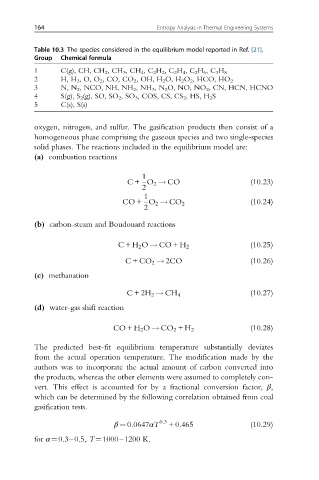
Table 10.3 The species considered in the equilibrium model reported in Ref. [21].
Group Chemical formula
1 C(g), CH, CH 2 ,CH 3 ,CH 4 ,C 2 H 2 ,C 2 H 4 ,C 2 H 6 ,C 3 H 8
2 H, H 2 ,O,O 2 , CO, CO 2 ,OH,H 2 O, H 2 O 2 , HCO, HO 2
3 N, N 2 , NCO, NH, NH 2 ,NH 3 ,N 2 O, NO, NO 2 , CN, HCN, HCNO
4 S(g), S 2 (g), SO, SO 2 ,SO 3 , COS, CS, CS 2 , HS, H 2 S
5 C(s), S(s)
oxygen, nitrogen, and sulfur. The gasification products then consist of a
homogeneous phase comprising the gaseous species and two single-species
solid phases. The reactions included in the equilibrium model are:
(a) combustion reactions
1
C+ O 2 ! CO (10.23)
2
1
CO + O 2 ! CO 2 (10.24)
2
(b) carbon-steam and Boudouard reactions
C+H 2 O ! CO + H 2 (10.25)
C+CO 2 ! 2CO (10.26)
(c) methanation
C+2H 2 ! CH 4 (10.27)
(d) water-gas shift reaction
(10.28)
CO + H 2 O ! CO 2 +H 2
The predicted best-fit equilibrium temperature substantially deviates
from the actual operation temperature. The modification made by the
authors was to incorporate the actual amount of carbon converted into
the products, whereas the other elements were assumed to completely con-
vert. This effect is accounted for by a fractional conversion factor, β,
which can be determined by the following correlation obtained from coal
gasification tests.
0:3
β ¼ 0:0647αT +0:465 (10.29)
for α¼0.3–0.5, T¼1000–1200 K.