Page 168 - Entrophy Analysis in Thermal Engineering Systems
P. 168
Entropy and chemical equilibrium 163
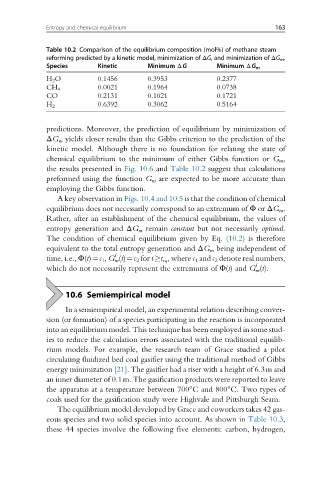
Table 10.2 Comparison of the equilibrium composition (mol%) of methane steam
reforming predicted by a kinetic model, minimization of ΔG, and minimization of ΔG m .
Species Kinetic Minimum ΔG Minimum ΔG m
H 2 O 0.1456 0.3953 0.2377
0.0021 0.1964 0.0738
CH 4
CO 0.2131 0.1021 0.1721
0.6392 0.3062 0.5164
H 2
predictions. Moreover, the prediction of equilibrium by minimization of
ΔG m yields closer results than the Gibbs criterion to the prediction of the
kinetic model. Although there is no foundation for relating the state of
chemical equilibrium to the minimum of either Gibbs function or G m ,
the results presented in Fig. 10.6 and Table 10.2 suggest that calculations
preformed using the function G m are expected to be more accurate than
employing the Gibbs function.
A key observation in Figs. 10.4 and 10.5 is that the condition of chemical
equilibrium does not necessarily correspond to an extremum of Φ or ΔG m .
Rather, after an establishment of the chemical equilibrium, the values of
entropy generation and ΔG m remain constant but not necessarily optimal.
The condition of chemical equilibrium given by Eq. (10.2) is therefore
equivalent to the total entropy generation and ΔG m being independent of
time, i.e., Φ(t)¼c 1 , G m (t)¼c 2 for t t eq , where c 1 and c 2 denote real numbers,
f
which do not necessarily represent the extremums of Φ(t) and G m (t).
f
10.6 Semiempirical model
In a semiempirical model, an experimental relation describing conver-
sion (or formation) of a species participating in the reaction is incorporated
into an equilibrium model. This technique has been employed in some stud-
ies to reduce the calculation errors associated with the traditional equilib-
rium models. For example, the research team of Grace studied a pilot
circulating fluidized bed coal gasifier using the traditional method of Gibbs
energy minimization [21]. The gasifier had a riser with a height of 6.3m and
an inner diameter of 0.1m. The gasification products were reported to leave
the apparatus at a temperature between 700°C and 800°C. Two types of
coals used for the gasification study were Highvale and Pittsburgh Seam.
The equilibrium model developed by Grace and coworkers takes 42 gas-
eous species and two solid species into account. As shown in Table 10.3,
these 44 species involve the following five elements: carbon, hydrogen,