Page 171 - Entrophy Analysis in Thermal Engineering Systems
P. 171
166 Entropy Analysis in Thermal Engineering Systems
1000
Measured CH 4
Measured H
100 2
10 H
Molar content (%) 0.1 1 2
0.01
CH 4
0.001
0.0001
0.3 0.35 0.4 0.45 0.5 0.55 0.6
Air ratio (−)
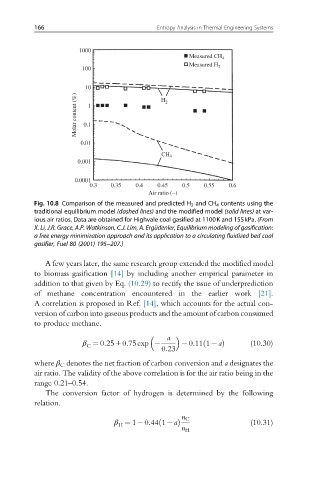
Fig. 10.8 Comparison of the measured and predicted H 2 and CH 4 contents using the
traditional equilibrium model (dashed lines) and the modified model (solid lines) at var-
ious air ratios. Data are obtained for Highvale coal gasified at 1100K and 155kPa. (From
X. Li, J.R. Grace, A.P. Watkinson, C.J. Lim, A. Erg€ udenler, Equilibrium modeling of gasification:
a free energy minimization approach and its application to a circulating fluidized bed coal
gasifier, Fuel 80 (2001) 195–207.)
A few years later, the same research group extended the modified model
to biomass gasification [14] by including another empirical parameter in
addition to that given by Eq. (10.29) to rectify the issue of underprediction
of methane concentration encountered in the earlier work [21].
A correlation is proposed in Ref. [14], which accounts for the actual con-
version of carbon into gaseous products and the amount of carbon consumed
to produce methane.
a
ð
β ¼ 0:25 + 0:75exp 0:11 1 aÞ (10.30)
C
0:23
where β C denotes the net fraction of carbon conversion and a designates the
air ratio. The validity of the above correlation is for the air ratio being in the
range 0.21–0.54.
The conversion factor of hydrogen is determined by the following
relation.
n C
β ¼ 1 0:44 1 að Þ (10.31)
H
n H