Page 175 - Entrophy Analysis in Thermal Engineering Systems
P. 175
170 Entropy Analysis in Thermal Engineering Systems
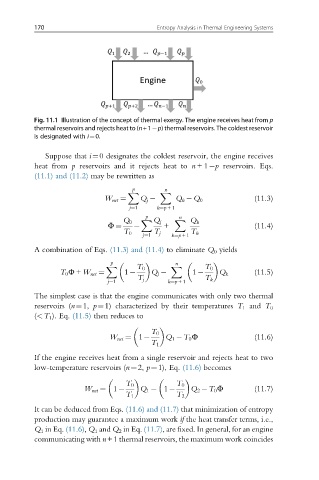
Fig. 11.1 Illustration of the concept of thermal exergy. The engine receives heat from p
thermal reservoirs and rejects heat to (n+1 p) thermal reservoirs. The coldest reservoir
is designated with i¼0.
Suppose that i¼0 designates the coldest reservoir, the engine receives
heat from p reservoirs and it rejects heat to n+ 1 p reservoirs. Eqs.
(11.1) and (11.2) may be rewritten as
p n
X X
W net ¼ Q j Q k Q 0 (11.3)
j¼1 k¼p +1
p
n
Q 0 X Q j X
Φ ¼ + Q k (11.4)
T 0
j¼1 T j k¼p +1 T k
A combination of Eqs. (11.3) and (11.4) to eliminate Q 0 yields
p
n
T 0 T 0
X X
T 0 Φ + W net ¼ 1 Q j 1 Q k (11.5)
j¼1 T j k¼p +1 T k
The simplest case is that the engine communicates with only two thermal
reservoirs (n¼1, p¼1) characterized by their temperatures T 1 and T 0
(<T 1 ). Eq. (11.5) then reduces to
T 0
W net ¼ 1 Q 1 T 0 Φ (11.6)
T 1
If the engine receives heat from a single reservoir and rejects heat to two
low-temperature reservoirs (n¼2, p¼1), Eq. (11.6) becomes
T 0 T 0
W net ¼ 1 Q 1 1 Q 2 T 0 Φ (11.7)
T 1 T 2
It can be deduced from Eqs. (11.6) and (11.7) that minimization of entropy
production may guarantee a maximum work if the heat transfer terms, i.e.,
Q 1 in Eq. (11.6), Q 1 and Q 2 in Eq. (11.7), are fixed. In general, for an engine
communicating with n+1 thermal reservoirs, the maximum work coincides