Page 180 - Entrophy Analysis in Thermal Engineering Systems
P. 180
Exergy 175
ch
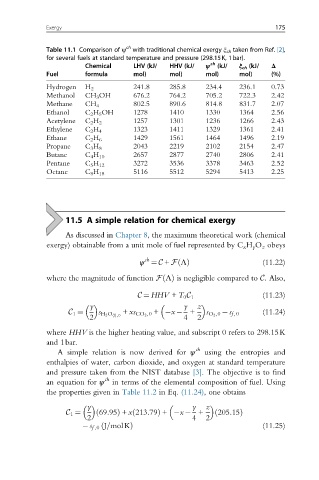
Table 11.1 Comparison of ψ with traditional chemical exergy ξ ch taken from Ref. [2],
for several fuels at standard temperature and pressure (298.15K, 1bar).
Chemical LHV (kJ/ HHV (kJ/ ψ ch (kJ/ ξ ch (kJ/ Δ
Fuel formula mol) mol) mol) mol) (%)
Hydrogen H 2 241.8 285.8 234.4 236.1 0.73
Methanol CH 3 OH 676.2 764.2 705.2 722.3 2.42
Methane CH 4 802.5 890.6 814.8 831.7 2.07
Ethanol C 2 H 5 OH 1278 1410 1330 1364 2.56
Acetylene C 2 H 2 1257 1301 1236 1266 2.43
Ethylene C 2 H 4 1323 1411 1329 1361 2.41
Ethane C 2 H 6 1429 1561 1464 1496 2.19
Propane C 3 H 8 2043 2219 2102 2154 2.47
Butane C 4 H 10 2657 2877 2740 2806 2.41
Pentane C 5 H 12 3272 3536 3378 3463 2.52
Octane C 8 H 18 5116 5512 5294 5413 2.25
11.5 A simple relation for chemical exergy
As discussed in Chapter 8, the maximum theoretical work (chemical
exergy) obtainable from a unit mole of fuel represented by C x H y O z obeys
ψ ¼C + F ΛðÞ (11.22)
ch
where the magnitude of function F ΛðÞ is negligible compared to C. Also,
C¼ HHV + T 0 C 1 (11.23)
y y z
C 1 ¼ s H 2 O lðÞ,0 + xs CO 2 ,0 + x + s O 2 ,0 s f ,0 (11.24)
2 4 2
where HHV is the higher heating value, and subscript 0 refers to 298.15K
and 1bar.
A simple relation is now derived for ψ ch using the entropies and
enthalpies of water, carbon dioxide, and oxygen at standard temperature
and pressure taken from the NIST database [3]. The objective is to find
an equation for ψ in terms of the elemental composition of fuel. Using
ch
the properties given in Table 11.2 in Eq. (11.24), one obtains
y y z
ð
C 1 ¼ ð 69:95Þ + x 213:79Þ + x + ð 205:15Þ
2 4 2
s f ,0 J=mol Kð Þ (11.25)