Page 39 - Entrophy Analysis in Thermal Engineering Systems
P. 39
30 Entropy Analysis in Thermal Engineering Systems
efficiency are not entirely convincing. The Carnot principles—also called
Carnot corollaries or propositions—state that
I. the efficiency of an irreversible engine cannot exceed that of the revers-
ible one operating between the same thermal reservoirs,
II. all reversible engines operating between the same two thermal reservoirs
possess the same efficiency.
3.2.1 Proof of corollaries
We now briefly review the method of demonstration of Carnot’s first cor-
ollary, which can be found in nearly all engineering thermodynamics texts.
The method was originally presented by Carnot himself to show that his
ideal engine is the most efficient among all engines operating between
two fixed temperature thermal reservoirs.
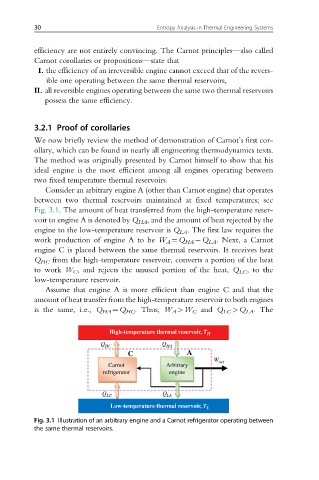
Consider an arbitrary engine A (other than Carnot engine) that operates
between two thermal reservoirs maintained at fixed temperatures; see
Fig. 3.1. The amount of heat transferred from the high-temperature reser-
voir to engine A is denoted by Q HA , and the amount of heat rejected by the
engine to the low-temperature reservoir is Q LA . The first law requires the
work production of engine A to be W A ¼Q HA Q LA . Next, a Carnot
engine C is placed between the same thermal reservoirs. It receives heat
Q HC from the high-temperature reservoir, converts a portion of the heat
to work W C , and rejects the unused portion of the heat, Q LC , to the
low-temperature reservoir.
Assume that engine A is more efficient than engine C and that the
amount of heat transfer from the high-temperature reservoir to both engines
is the same, i.e., Q HA ¼Q HC . Thus, W A >W C and Q LC >Q LA . The
High-temperature thermal reservoir, T H
Q HC Q HA
C A
W net
Carnot Arbitrary
refrigerator engine
Q LC Q LA
Low-temperature thermal reservoir, T L
Fig. 3.1 Illustration of an arbitrary engine and a Carnot refrigerator operating between
the same thermal reservoirs.