Page 142 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 142
128 Principles and Methods
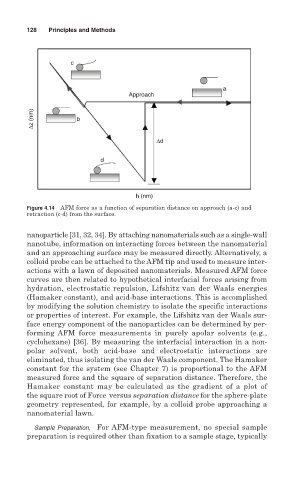
c
a
Approach
∆z (nm) b
∆d
d
h (nm)
Figure 4.14 AFM force as a function of separation distance on approach (a-c) and
retraction (c-d) from the surface.
nanoparticle [31, 32, 34]. By attaching nanomaterials such as a single-wall
nanotube, information on interacting forces between the nanomaterial
and an approaching surface may be measured directly. Alternatively, a
colloid probe can be attached to the AFM tip and used to measure inter-
actions with a lawn of deposited nanomaterials. Measured AFM force
curves are then related to hypothetical interfacial forces arising from
hydration, electrostatic repulsion, Lifshitz van der Waals energies
(Hamaker constant), and acid-base interactions. This is accomplished
by modifying the solution chemistry to isolate the specific interactions
or properties of interest. For example, the Lifshitz van der Waals sur-
face energy component of the nanoparticles can be determined by per-
forming AFM force measurements in purely apolar solvents (e.g.,
cyclohexane) [36]. By measuring the interfacial interaction in a non-
polar solvent, both acid-base and electrostatic interactions are
eliminated, thus isolating the van der Waals component. The Hamaker
constant for the system (see Chapter 7) is proportional to the AFM
measured force and the square of separation distance. Therefore, the
Hamaker constant may be calculated as the gradient of a plot of
the square root of Force versus separation distance for the sphere-plate
geometry represented, for example, by a colloid probe approaching a
nanomaterial lawn.
Sample Preparation. For AFM-type measurement, no special sample
preparation is required other than fixation to a sample stage, typically