Page 199 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 199
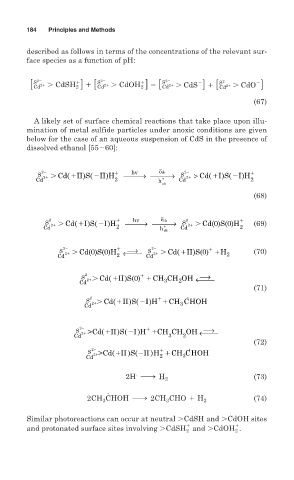
184 Principles and Methods
described as follows in terms of the concentrations of the relevant sur-
face species as a function of pH:
[ S Cd 21 . CdSH 2 ] 1 [ S Cd 21 . CdOH 2 ] 5 [ Cd . CdS ] 1 [ Cd . CdO ]
22
22
22
22
S
1
1
2
S
2
21
21
(67)
A likely set of surface chemical reactions that take place upon illu-
mination of metal sulfide particles under anoxic conditions are given
below for the case of an aqueous suspension of CdS in the presence of
dissolved ethanol [55 60]:
→
→
2 hv ecb 2
S 2 Cd( II)S( II)H ⎯⎯⎯ ⎯⎯⎯ S > Cd(I)S(I)H
Cd 2 h + Cd 2 2
vb
(68)
→
2 hv e cb 2
→
S 2 Cd( I)S( I)H ⎯⎯⎯ ⎯⎯⎯ S 2 Cd(0)S(0)H (69)
+
d
Cd 2 h vb Cd 2
⎯→
2 2
)
S 2 Cd(0)S(0)H ←⎯⎯ S 2 Cd( II)S(0) H (70)
Cd 2 Cd 2
2 ⎯→
S 2 Cd( II)S(0) CH CH OH ←⎯⎯
Cd 3 2
(71)
.
2
S 2 Cdd( II)S( I)H CH CHOH
Cd 3
2 ⎯→
S 2 >Cd( II)S( I )H CH CH OH ←⎯⎯
Cd 3 2
(72)
.
2
S 2 >>Cd( II )S( II )H CH CHOH
Cd 2 3
.
2H h H 2 (73)
.
2CH CHOH h 2CH CHO 1 H 2 (74)
3
3
Similar photoreactions can occur at neutral CdSH and CdOH sites
and protonated surface sites involving CdSH 2 and CdOH 2 .