Page 198 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 198
Reactive Oxygen Species Generation on Nanoparticulate Material 183
The electronic levels of nanoparticulate or quantum-sized CdS (Q-CdS)
can be tuned by changing or controlling particle size without changing the
chemical composition. For example, Hoffman et al. [35] found an increase
in quantum efficiency for photo-polymerization of methylmethacrylate
with a corresponding decrease in particle size using Q-CdS.
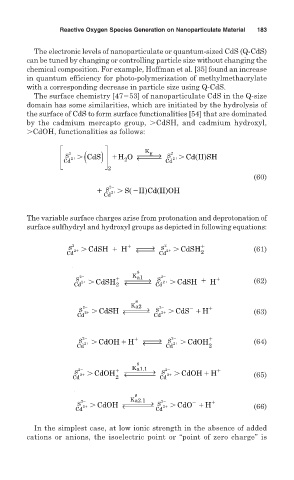
The surface chemistry [47 53] of nanoparticulate CdS in the Q-size
domain has some similarities, which are initiated by the hydrolysis of
the surface of CdS to form surface functionalities [54] that are dominated
by the cadmium mercapto group, CdSH, and cadmium hydroxyl,
CdOH, functionalities as follows:
⎡ ⎤ K
→
⎯⎯⎯
⎢ S 2 ( H O ←⎯⎯ S 2 2 Cd(II)SH
2 CdS) ⎥
⎯
H
2
⎢ ⎣ Cd ⎥ 2 ⎦ Cd
(60)
S 2 2 S( II)Cd(II)OH
Cd
The variable surface charges arise from protonation and deprotonation of
surface sulfhydryl and hydroxyl groups as depicted in following equations:
⎯→
⎯
2 2
⎯
S 2 CdSH H ←⎯ S 2 CdSH (61)
Cd Cd 2
s
2 K a1 2
→
S 2 CdSH ⎯⎯⎯ S 2 CdSH (62)
Cd 2 ←⎯⎯ ⎯ Cd H
s
→
Ka2
2 ⎯⎯⎯ 2
S 2 CdSH ←⎯⎯ ⎯ S 2 CdS H (63)
Cd Cd
⎯
⎯→
2 2
⎯
S 2 CdOH H ←⎯ S 2 CdOH (64)
Cd Cd 2
s
⎯⎯⎯⎯
→
2 Ka1.1 2
⎯
S 2 CdOH ←⎯⎯⎯ S 2 CdOH H
H
Cd 2 Cd (65)
s
Ka2.1
→
2 ⎯⎯⎯⎯ 2
⎯
S 2 CdOH ←⎯⎯⎯ S 2 CdO H
Cd Cd (66)
In the simplest case, at low ionic strength in the absence of added
cations or anions, the isoelectric point or “point of zero charge” is