Page 200 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 200
Reactive Oxygen Species Generation on Nanoparticulate Material 185
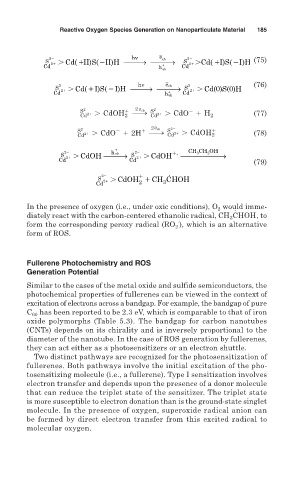
→
2 hv e cb 2
→
S 2 Cd( II)S( II)H ⎯⎯⎯ ⎯⎯⎯ S 2 Cd(I)S(I)H (75)
+
Cd h vb Cd d
→
⎯⎯
2 hv e cb 2 (76)
S 2 Cd( I)S( I)H ⎯→ ⎯⎯⎯ S 2 Cd(0)S(0)H
+
Cd h vb Cd
S 22 1 2 e cb S 22 21 . CdO 1 H
2
Cd 21 . CdOH 2 h Cd 2 (77)
2
S 22 21 . CdO 1 2H 1 2 e cb S 22 1 (78)
Cd h Cd 21 . CdOH 2
+ CH CH OH
→
→ →
2 2 . 3 2
hvb
S 2 CdOH ⎯⎯⎯ S 2 CdOH ⎯⎯⎯⎯⎯⎯
Cd Cd (79)
.
2
S 2 CdOH CH CHOH
Cd 2 3
would imme-
In the presence of oxygen (i.e., under oxic conditions), O 2 .
diately react with the carbon-centered ethanolic radical, CH 3 CHOH , to
.
form the corresponding peroxy radical (RO ), which is an alternative
2
form of ROS.
Fullerene Photochemistry and ROS
Generation Potential
Similar to the cases of the metal oxide and sulfide semiconductors, the
photochemical properties of fullerenes can be viewed in the context of
excitation of electrons across a bandgap. For example, the bandgap of pure
C 60 has been reported to be 2.3 eV, which is comparable to that of iron
oxide polymorphs (Table 5.3). The bandgap for carbon nanotubes
(CNTs) depends on its chirality and is inversely proportional to the
diameter of the nanotube. In the case of ROS generation by fullerenes,
they can act either as a photosensitizers or an electron shuttle.
Two distinct pathways are recognized for the photosensitization of
fullerenes. Both pathways involve the initial excitation of the pho-
tosensitizing molecule (i.e., a fullerene). Type I sensitization involves
electron transfer and depends upon the presence of a donor molecule
that can reduce the triplet state of the sensitizer. The triplet state
is more susceptible to electron donation than is the ground-state singlet
molecule. In the presence of oxygen, superoxide radical anion can
be formed by direct electron transfer from this excited radical to
molecular oxygen.