Page 193 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 193
178 Principles and Methods
opposing charge acceptor. The charge trapping capacity of the surface
eventually decreases following the complete recombination of a large
number of free carriers. The charge carrier recombination rate increases
with increasing surface hydration, indicating that surface hydroxylation
assists annihilation reactions by allowing irreversible electron trap-
ping. A surface electron trap state observed at 0.42 eV may be respon-
sible for the mediating the annihilation mechanism.
A number of alternative spectroscopic techniques have been applied
and other
to characterize the lifetime of the mobile electrons in TiO 2
semiconductors. Martin et al. [32, 33] used microwave frequency and
Herrmann et al. [34] used radio frequency spectroscopy as a probe tech-
nique after laser excitation and determined a broad range of lifetimes
for the various trapping states from microseconds to milliseconds.
Most metal oxide and mixed-metal oxide semiconductor surface chem-
istry is dominated by hydroxyl groups when in the presence of water or
humid air. For example, the metal niobates, LiNbO 3 and KNbO 3 , are
widely used electro-optic and photorefractive materials that depend on
the activation of surface protons (i.e., protons bound in hydroxyl ions,
OH). The hydroxyl bound protons have activation energies in the range
of 1 eV for mobility in LiNbO 3 and KNbO 3 crystals. The corresponding
surface hydration in KNbO 3 leads to the following reactions:
⎯
⎯→
Nb(V)ONb(V) H O ←⎯⎯ 2 Nb(V)OH (38)
2
2.5
in water
Q-sized nanoparticulate TiO 2
2.4 380 nm hn on
380 nm hn on
2.3
pH
2.2
Light off
2.1
0 20 50 75 100 125 150 175
Time (nm)
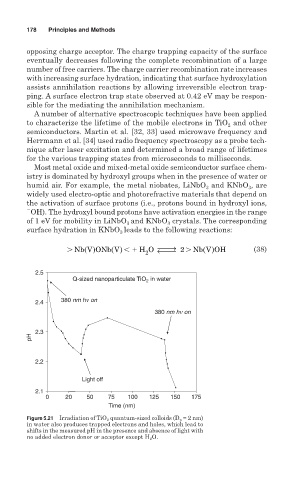
Figure 5.21 Irradiation of TiO 2 quantum-sized colloids (D p = 2 nm)
in water also produces trapped electrons and holes, which lead to
shifts in the measured pH in the presence and absence of light with
no added electron donor or acceptor except H 2 O.