Page 192 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 192
Reactive Oxygen Species Generation on Nanoparticulate Material 177
8
7
a
b Time
6 c d Relative log (norm[signal-base] / (K-M)
0.5
5 a
K-M 4 3.5 Log(E/cm ) 3.1 b
3.3
–1
c
3
d
2
1 e
0
4000 3000 2000 1000
E/cm –1
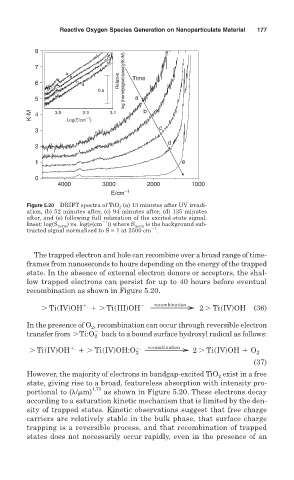
Figure 5.20 DRIFT spectra of TiO 2 (a) 13 minutes after UV irradi-
ation, (b) 52 minutes after, (c) 94 minutes after, (d) 135 minutes
after, and (e) following full relaxation of the excited-state signal.
1
n
Inset: log(S norm ) vs. log( (cm )) where S norm is the background sub-
1
tracted signal normalized to S = 1 at 2500 cm .
The trapped electron and hole can recombine over a broad range of time-
frames from nanoseconds to hours depending on the energy of the trapped
state. In the absence of external electron donors or acceptors, the shal-
low trapped electrons can persist for up to 40 hours before eventual
recombination as shown in Figure 5.20.
recombination
2
. TisIVdOH 1. 1. TisIIIdOH h 2 . TisIVdOH (36)
In the presence of O 2 , recombination can occur through reversible electron
2.
transfer from .Ti:O 2 back to a bound surface hydroxyl radical as follows:
recombination
2.
. TisIVdOH 1. 1. TisIVdOH:O 2 h 2 . TisIVdOH 1 O 2
(37)
However, the majority of electrons in bandgap-excited TiO exist in a free
2
state, giving rise to a broad, featureless absorption with intensity pro-
1.73
portional to ( / m) as shown in Figure 5.20. These electrons decay
according to a saturation kinetic mechanism that is limited by the den-
sity of trapped states. Kinetic observations suggest that free charge
carriers are relatively stable in the bulk phase, that surface charge
trapping is a reversible process, and that recombination of trapped
states does not necessarily occur rapidly, even in the presence of an