Page 331 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 331
316 Environmental Applications of Nanomaterials
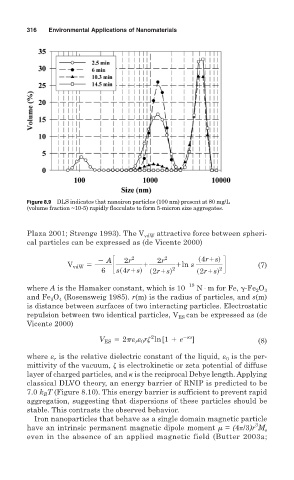
Figure 8.9 DLS indicates that nanoiron particles (100 nm) present at 80 mg/L
(volume fraction ~10-5) rapidly flocculate to form 5-micron size aggregates.
Plaza 2001; Strenge 1993). The V vdW attractive force between spheri-
cal particles can be expressed as (de Vicente 2000)
2 A 2r 2 2r 2 s4r1sd
V vdW 5 c 1 1ln s d (7)
6 ss4r1sd s2r1sd 2 s2r1sd 2
where A is the Hamaker constant, which is 10 19 N ⋅ m for Fe, -Fe O 3
2
and Fe O (Rosensweig 1985). r(m) is the radius of particles, and s(m)
4
3
is distance between surfaces of two interacting particles. Electrostatic
repulsion between two identical particles, V ES can be expressed as (de
Vicente 2000)
V ES 5 2pe e rz ln [1 1 e 2ks ] (8)
2
r 0
where is the relative dielectric constant of the liquid, is the per-
r
0
mittivity of the vacuum, is electrokinetic or zeta potential of diffuse
layer of charged particles, and k is the reciprocal Debye length. Applying
classical DLVO theory, an energy barrier of RNIP is predicted to be
T (Figure 8.10). This energy barrier is sufficient to prevent rapid
7.0 k B
aggregation, suggesting that dispersions of these particles should be
stable. This contrasts the observed behavior.
Iron nanoparticles that behave as a single domain magnetic particle
3
have an intrinsic permanent magnetic dipole moment m = (4 /3)r M s
even in the absence of an applied magnetic field (Butter 2003a;